Fluorescence Digital Image Gallery
Madin-Darby Canine Kidney Epithelial Cells (MDCK Line)
Derived by S. H. Madin and N. B. Darby from the kidney tissue of an adult female cocker spaniel, the MDCK cell line originated in September 1958. Since that time, the cells have been widely utilized to investigate the processing of beta-amyloid precursor protein, as well as the sorting of its proteolytic products.
The morphology of the MDCK cell line is epithelial, and the cells are positive for keratin by immunoperoxidase staining. Viruses that MDCK cells are susceptible to include vesicular stomatitis (Indiana strain), vaccinia, coxsackievirus B5, reovirus 2 and 3, adenovirus 4 and 5, vesicular exanthema of swine, and infectious canine hepatitis. The cells exhibit resistance to coxsackievirus B3 and B4 as well as poliovirus 2, and are negative for reverse transcriptase.
The MDCK line is commonly used as a general model for epithelial cells, which comprise the type of tissue known as epithelium. Chiefly found covering the internal organs and other surfaces of the body, epithelium is comprised of tightly packed cells that are organized into sheets. These cells secrete an extracellular matrix called the basal lamina at their base, which helps anchor the epithelial tissue to adjacent tissues. Epithelial cells also lack direct access to blood vessels and must, therefore, obtain oxygen and nutrients through diffusion, the same way that they are forced to rid themselves of metabolic waste products. Epithelia function in a variety of mechanisms, including protection, absorption, sensory reception, and secretion. The epithelial cells of the kidneys play a key role in the temporary storage and subsequent secretion of excretory materials.
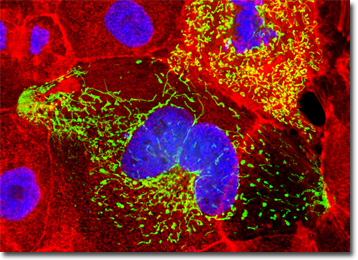
The culture of Madin-Darby canine kidney cells presented in the digital image above was labeled with DAPI and Alexa Fluor 568 conjugated to phalloidin, which target DNA in the cell nucleus and the F-actin cytoskeletal network, respectively. In addition, the cells were transfected with a pEYFP-Mitochondria (enhanced yellow fluorescent protein) chimeric plasmid subcellular localization vector. Images were recorded in grayscale with a QImaging Retiga Fast-EXi camera system coupled to an Olympus BX-51 microscope equipped with bandpass emission fluorescence filter optical blocks provided by Omega Optical. During the processing stage, individual image channels were pseudocolored with RGB values corresponding to each of the fluorophore emission spectral profiles.
Additional Fluorescence Images of Madin-Darby Canine Kidney (MDCK) Cells
MDCK Epithelial Cells with MitoTracker Red CMXRos, Alexa Fluor 488, and DAPI - An adherent culture of Madin-Darby canine kidney cells was labeled for the intracellular mitochondrial network and for filamentous actin with MitoTracker Red CMXRos and Alexa Fluor 488 conjugated to phalloidin, respectively. The ultraviolet-absorbing probe DAPI was utilized to counterstain DNA.
Targeting Peroxisomes and Clathrin Proteins in Madin-Darby Kidney Cells with Immunofluorescence - In this section, the featured culture of MDCK cells was immunofluorescently labeled with primary anti-clathrin (heavy chain) mouse monoclonal antibodies followed by goat anti-mouse Fab fragments conjugated to the cyanine dye Cy3 in order to target the cytoskeletal network. Additionally, peroxisomes present in the culture were immunofluorescently labeled with Cy2 conjugated to goat secondary antibodies directed against rabbit anti-PMP 70 (peroxisomal membrane protein 70) primary antibodies. Nuclei were counterstained with Hoechst 33342.
Enhanced Yellow Protein Subcellular Localization of Mitochondria in MDCK Cell Cultures - A culture of Madin-Darby canine kidney epithelial cells was transfected with a pEYFP-Mitochondria (enhanced yellow fluorescent protein) chimeric plasmid subcellular localization vector. The cells were also stained with SYTOX Orange and Alexa Fluor 350 conjugated to phalloidin, targeting DNA and the cytoskeletal filamentous actin network, respectively.
Immunofluorescence Targeting of the Histones and Golgi Complex in Canine Kidney Epithelial Cell Cultures - Madin-Darby canine kidney cells were fixed with paraformaldehyde, permeabilized, and treated with a mixture of rabbit (anti-giantin) and mouse (anti-histones; pan) primary antibodies, followed by secondary antibodies conjugated to Alexa Fluor 488 and Texas Red, respectively. The secondary antibody cocktail also contained Alexa Fluor 350 conjugated to phalloidin, designed to simultaneously target the filamentous actin network.
Double Immunofluorescence of Nuclear Pore Complex Proteins and Tight Junctions in Madin-Darby Canine Kidney Cells - Epithelial cell tight junctions and nuclear pore complex proteins were simultaneously imaged in MDCK cells with a cocktail of mouse anti-NPCP and rabbit anti-ZO-3 primary antibodies, followed by goat anti-mouse and anti-rabbit secondary antibodies conjugated to Alexa Fluor 488 and Alexa Fluor 568, respectively.
The Mitochondrial Network in MDCK Cells - An adherent monolayer culture of Madin-Darby canine kidney cells was immunofluorescently labeled with primary mouse anti-oxphos complex V inhibitor protein antibodies, followed by goat anti-mouse Fab fragments conjugated to fluorescein. The culture was subsequently stained with Alexa Fluor 568 conjugated to phalloidin to reveal details of the filamentous actin network, and DAPI for DNA in the nucleus.
MDCK Cells with Wheat Germ Agglutinin - Lectins are a specialized class of plant proteins that bind to specific carbohydrate groups attached to proteins or residing in cell membranes. A prominent member of this group, wheat germ agglutinin is often used to localize the Golgi complex in fluorescence labeling experiments. The culture illustrated in this section was labeled with wheat germ agglutinin conjugated to Texas Red, as well as Alexa Fluor 488 conjugated to phalloidin and DAPI (targeting DNA in the nucleus).
Targeting Mitochondria with Fluorescent Proteins in Canine Kidney Cell Cultures - A semi-confluent culture of MDCK cells was transiently transfected with a plasmid chimera containing the coding sequence for enhanced yellow fluorescent protein (EYFP) fused to the mitochondrial targeting sequence from subunit VIII of human cytochrome C oxidase. After fixation and permeabilization, the cells were counterstained for filamentous actin with Alexa Fluor 546 conjugated to phalloidin and for DNA with the nuclear-specific dye, DAPI.
Tight Junctions in Madin-Darby Canine Kidney Cell Cultures - The tight junction protein ZO-3 was visualized by immunofluorescence in a confluent culture of MDCK cells. Rabbit anti-ZO-3 antibodies targeting the protein were tagged with goat-anti rabbit secondary Fab fragments conjugated to the cyanine dye, Cy3. The nuclei were counterstained with the DNA-specific fluorophore DAPI.
MDCK Adherent Cell Cultures with Texas Red, Alexa Fluor 488, and Alexa Fluor 350 - In a double immunofluorescence labeling experiment, a culture of MDCK cells was treated with a cocktail of mouse anti-histones (pan) and rabbit anti-PMP 70 (peroxisomal membrane protein) primary antibodies. The target proteins were subsequently visualized with goat anti-mouse and anti-rabbit secondary antibodies conjugated to Texas Red and Alexa Fluor 488, respectively. The filamentous actin cytoskeletal network was counterstained with Alexa Fluor 350 conjugated to phalloidin.
Classical Staining Patterns in Canine Kidney Epithelial Cells - The now traditional and popular combination of MitoTracker Red CMXRos, Alexa Fluor 488 conjugated to phalloidin, and Hoechst 33342 was used to triple-label an adherent culture of MDCK cells. Note the unusual filamentous actin network, and the large number of mitochondria surrounding the nuclei in these cells.
Subcellular Localization of Mitochondria with Fluorescent Proteins in MDCK Cells - After fixation and permeabilization, the culture of MDCK cells presented in this section was labeled with propidium iodide and Alexa Fluor 350 conjugated to phalloidin, which target DNA and filamentous actin, respectively. In addition, the cells were first transfected with a pEYFP-Mitochondria plasmid subcellular localization vector, thus localizing a yellow fluorescent protein tag to the intracellular mitochondrial network.
The Extensive Tubulin Network in Madin-Darby Canine Kidney Epithelial Cells - Microtubules were stained using immunofluorescence by treating a fixed and permeabilized culture of MDCK cells with mouse-anti-alpha-tubulin primary antibodies followed by goat anti-mouse antibodies conjugated to Alexa Fluor 568. Nuclei were counterstained with SYTOX Green. Although not imaged in this section, the culture was also labeled with Alexa Fluor 350 conjugated to phalloidin.
Simultaneous Visualization of Clathrin, Peroxisomes, and Nuclei in MDCK Cell Cultures - The culture of MDCK cells presented in this section was immunofluorescently labeled with primary anti-clathrin (heavy chain) mouse monoclonal antibodies followed by goat anti-mouse Fab fragments conjugated to the cyanine dye Cy3 in order to target the cytoskeletal network. In addition, peroxisomes present in the culture were immunofluorescently labeled with Cy2 conjugated to goat secondary antibodies directed against rabbit anti-PMP 70 (peroxisomal membrane protein 70) primary antibodies. Nuclei were counterstained with Hoechst 33342.
The Cytokeratin Intermediate Filament Network in Canine Kidney Epithelial Cells - Cytokeratin is a common and abundant component of the intermediate filament network in epithelial cells. The adherent culture in this section was immunofluorescently labeled with mouse anti-cytokeratin (pan) primary antibodies, followed by goat anti-mouse secondary antibodies conjugated to Marina Blue. Mitochondria were visualized with MitoTracker Red CMXRos and the nuclei were stained with SYTOX Green.
Madin-Darby Canine Kidney Cells with Alexa Fluor 488, Alexa Fluor 568, and Hoechst 33258 - Simultaneous localization of tight junctions and the nuclear pore complex proteins (NPCP) was performed with a double immunofluorescence experiment with MDCK cells using mouse anti-NPCP and rabbit anti-ZO-3 primary antibodies. The subcellular targets were visualized using goat anti-mouse and anti-rabbit secondary antibodies (IgG) conjugated to Alexa Fluor 488 and Alexa Fluor 568, respectively. DNA in the nuclei was counterstained using Hoechst 33258.
Immunofluorescent Labeling of the Mitochondrial Network in MDCK Cell Cultures - Madin-Darby canine kidney cells were immunofluorescently labeled with primary mouse anti-oxphos complex V inhibitor protein antibodies, followed by goat anti-mouse Fab fragments conjugated to fluorescein. The culture was subsequently stained with Alexa Fluor 568 conjugated to phalloidin to reveal details of the filamentous actin network, and DAPI for DNA in the nucleus.
MitoTracker Red CMXRos, Cy2, and Hoechst 33258 in Madin-Darby Canine Kidney Cells - A fixed and permeabilized adherent culture of Madin-Darby canine kidney cells was immunofluorescently labeled with primary mouse anti-cytokeratin antibodies, followed by goat anti-mouse Fab fragments conjugated to the cyanine dye, Cy2. Before fixation, the culture was treated for one hour with MitoTracker Red CMXRos, and after the antibody treatments, the nuclei were counterstained with Hoechst 33258.
Binding of Lectins to the Golgi Complex in MDCK Epithelial Cell Cultures - In order to visualize lectin binding to the Golgi complex in MDCK cells, an adherent culture was treated with wheat germ agglutinin conjugated to Oregon Green. The cells were subsequently counterstained with Alexa Fluor 568 conjugated to phalloidin to localize the filamentous actin network, and the nucleic acid stain DAPI to label DNA in the nucleus.
Adherent MDCK Cells with Alexa Fluor 488, Alexa Fluor 568, and Hoechst 33342 - Alexa Fluor dyes were employed to visualize distribution of clathrin and the peroxisomal membrane protein 70 (PMP 70) in a culture of Madin-Darby canine kidney epithelial cells. After the immunofluorescence reactions, the cells were counterstained with Hoechst 33342 to reveal the location of the nuclei.
Madin-Darby Canine Kidney Cells with MitoTracker Red CMXRos, Alexa Fluor 488, and DAPI - One of the most useful fluorophore combinations for visualizing internal cellular details includes MitoTracker Red CMXRos to target mitochondria, Alexa Fluor 488 conjugated to phalloidin for the filamentous actin network, and DAPI to localize the nuclei.
Visualizing the Microtubule and Actin Cytoskeletal Networks in MDCK Cell Cultures - In order to simultaneously visualize both the actin and tubulin networks in MDCK cells, a fixed and permeabilized culture was treated with mouse anti-alpha-tubulin primary antibodies, followed by a cocktail of goat anti-mouse secondary antibodies conjugated to Alexa Fluor 568 mixed together with Alexa Fluor 350 conjugated to phalloidin. Nuclei were counterstained with SYTOX Green.
Double Immunofluorescence of Nuclear Pore Complex Proteins and Tight Junctions in Madin-Darby Canine Kidney Cells - Epithelial cell tight junctions and nuclear pore complex proteins were simultaneously imaged in MDCK cells with a cocktail of mouse anti-NPCP and rabbit anti-ZO-3 primary antibodies, followed by goat anti-mouse and anti-rabbit secondary antibodies conjugated to Alexa Fluor 568 and Alexa Fluor 488, respectively. Nuclei were counterstained with Hoechst 33258.
Proximity of the Nucleus and Golgi Complex in Monolayer MDCK Cell Cultures - The close proximity between the Golgi complex and nuclei in Madin-Darby canine kidney cells was probed in a double immunofluorescence experiment with mouse anti-NPCP (nuclear pore complex protein) and rabbit anti-giantin (Golgi complex) primary antibodies. The antibody targets were visualized with goat secondary antibodies conjugated to Alexa Fluor 568 and Alexa Fluor 488, respectively, while the actin cytoskeletal framework was labeled with Alexa Fluor 350 conjugated to phalloidin.
BACK TO THE CULTURED CELLS FLUORESCENCE GALLERY
BACK TO THE FLUORESCENCE GALLERY
