Fluorescence Digital Image Gallery
Horse Dermal Fibroblast Cells (NBL-6)
|
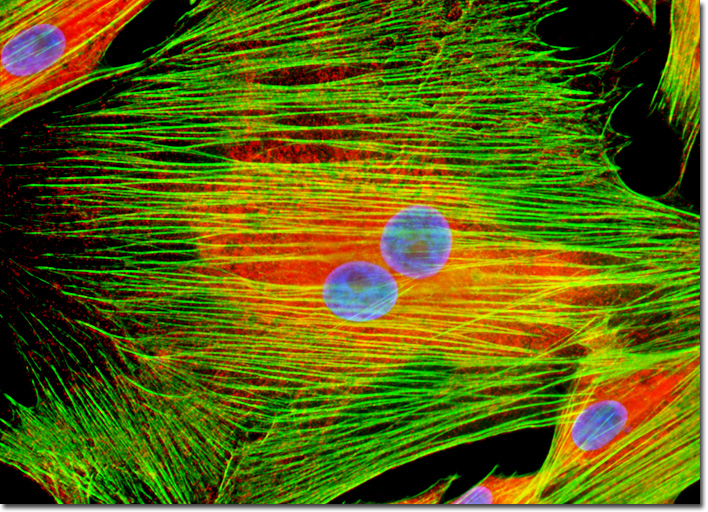
The endoplasmic reticulum (ER) is a membrane network present in all eukaryotic cells. Arranged into an extensive, net-like array of tubules and cisternae (sac-like structures), the endoplasmic reticulum accounts for more than 50 percent of the membranous material associated with a typical animal cell. The variously shaped components of the organelle are all interconnected, together sectioning off a highly convoluted internal region known as the ER lumen, which may comprise 10 percent or more of a cell's volume. The membrane of the endoplasmic reticulum acts as a selective barrier between this region and the surrounding cytosol, while simultaneously carrying out a number of other important functions. Chief among these activities is the production of proteins and lipids for use by other cellular components. Calreticulin is a calcium-binding protein resident in the membrane of the endoplasmic reticulum. The adherent culture of horse fibroblasts featured in the digital image above was immunofluorescently labeled with anti-calreticulin rabbit monoclonal primary antibodies followed by goat anti-rabbit secondary antibodies conjugated to Cy3. The cells were also stained with Alexa Fluor 488 conjugated to phalloidin and Hoechst 33342, which bind respectively with filamentous actin and DNA in the cell nucleus. Images were recorded in grayscale with a QImaging Retiga Fast-EXi camera system coupled to an Olympus BX-51 microscope equipped with bandpass emission fluorescence filter optical blocks provided by Omega Optical. During the processing stage, individual image channels were pseudocolored with RGB values corresponding to each of the fluorophore emission spectral profiles. |
© 1995-2025 by Michael W. Davidson and The Florida State University. All Rights Reserved. No images, graphics, software, scripts, or applets may be reproduced or used in any manner without permission from the copyright holders. Use of this website means you agree to all of the Legal Terms and Conditions set forth by the owners.
This website is maintained by our
|