Fluorescence Digital Image Gallery
Madin-Darby Ovine Kidney Epithelial Cells (MDOK)
|
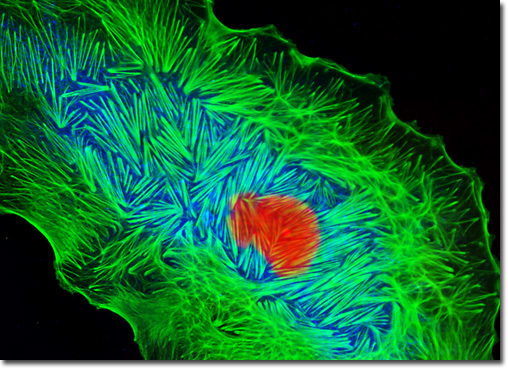
The defining attribute of rough endoplasmic reticulum (ER) is the presence of membrane-bound ribosomes along the cytoplasmic surface. These ribosomes synthesize proteins for concurrent translocation into the endoplasmic reticulum, whereas free ribosomes (those not attached to any membrane) synthesize the rest of the proteins encoded by the genome. As a growing protein is formed by a ribosome attached to the endoplasmic reticulum, it enters the lumen via a pore created by a protein resident in the organelle's membrane. During this process, the developing protein is folded into its characteristic three-dimensional structure. In certain specialized cell types, such as the pancreatic cells that emit insulin, these proteins are subsequently released in transport vesicles from endoplasmic reticulum exit sites, often traveling to the Golgi complex. Rough endoplasmic reticulum is not only important for the formation of secretory proteins, however. This region of the endoplasmic reticulum also generates proteins intended for use in membrane formation as well as phospholipids that are retained in the ER membrane. The adherent monolayer culture of Madin-Darby ovine kidney cells illustrated above was fixed, permeabilized, and treated with a mixture of concanavalin A conjugated to Alexa Fluor 350 and phalloidin conjugated to Alexa Fluor 488 to target the endoplasmic reticulum and filamentous actin network. After washing, the cells were counterstained with SYTOX Orange to visualize the nuclei. Images were recorded in grayscale with a QImaging Retiga Fast-EXi camera system coupled to an Olympus BX-51 microscope equipped with bandpass emission fluorescence filter optical blocks provided by Omega Optical. During the processing stage, individual image channels were pseudocolored with RGB values corresponding to each of the fluorophore emission spectral profiles. |
© 1995-2025 by Michael W. Davidson and The Florida State University. All Rights Reserved. No images, graphics, software, scripts, or applets may be reproduced or used in any manner without permission from the copyright holders. Use of this website means you agree to all of the Legal Terms and Conditions set forth by the owners.
This website is maintained by our
|