Fluorescence Digital Image Gallery
Madin-Darby Ovine Kidney Epithelial Cells (MDOK)
|
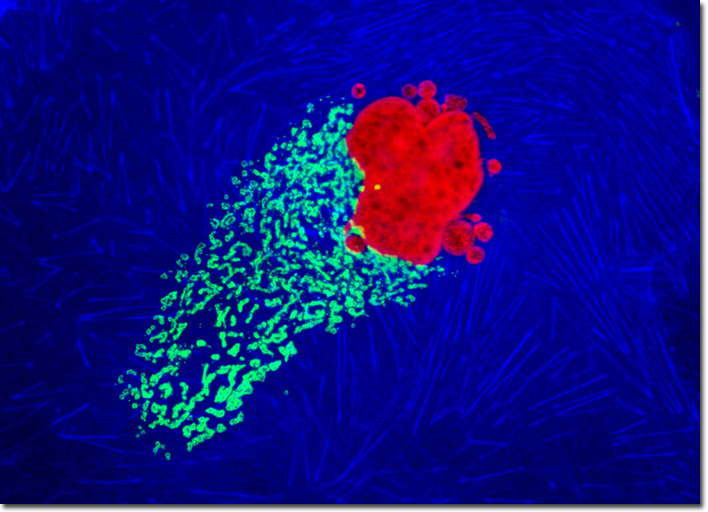
The generalized procedure for immunofluorescence labeling of adherent cell cultures involves fixation of the cells with a suitable reagent, such as paraformaldehyde, glutaraldehyde, acetone, or methanol. The organic solvents serve double duty by also removing most of the plasma membrane components, thus exposing the interior of the cell to antibodies and other non-permeant reagents. Cell cultures fixed with aldehydes require a permeabilization step with a detergent. Triton X-100 is a popular permeabilizing agent, as are saponin, Tween, and sodium dodecyl sulfate (SDS). After permeabilization, cells are treated with a primary antibody (or a mixture of two different antibodies from different hosts) followed by a fluorophore conjugated secondary antibody. In some cases, the primary antibody is labeled with a fluorophore. After immunostaining, the cells can be counterstained with other reagents to visual structural details not revealed by the fluorescent antibodies. In the double immunofluorescence experiment presented above, a culture of Madin-Darby ovine kidney cells was fixed, permeabilized, and blocked with 10-percent goat serum before being treated with a cocktail of mouse anti-histones (pan) and rabbit anti-giantin primary antibodies. The target organelles were visualized with goat anti-mouse and anti-rabbit secondary antibodies (IgG) conjugated to Texas Red and Alexa Fluor 488, respectively. The filamentous actin cytoskeletal network was imaged with Alexa Fluor 350 conjugated to phalloidin. Images were recorded in grayscale with a QImaging Retiga Fast-EXi camera system coupled to an Olympus BX-51 microscope equipped with bandpass emission fluorescence filter optical blocks provided by Omega Optical. During the processing stage, individual image channels were pseudocolored with RGB values corresponding to each of the fluorophore emission spectral profiles. |
© 1995-2025 by Michael W. Davidson and The Florida State University. All Rights Reserved. No images, graphics, software, scripts, or applets may be reproduced or used in any manner without permission from the copyright holders. Use of this website means you agree to all of the Legal Terms and Conditions set forth by the owners.
This website is maintained by our
|