Fluorescence Digital Image Gallery
Madin-Darby Canine Kidney Epithelial Cells (MDCK)
|
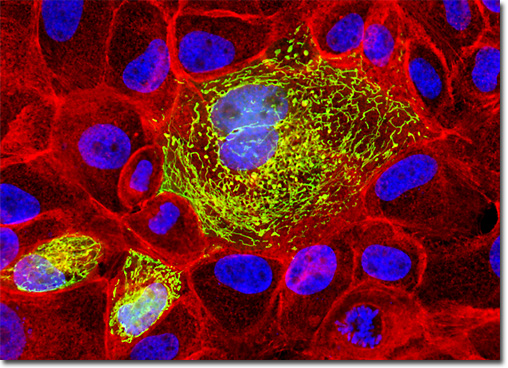
Localization of specific peptides, proteins, and macromolecular complexes to the mitochondria in mammalian cells is generally accomplished through the use of peptide signals that mediate transport to the organelle. Recombinant plasmids have been constructed that contain a fusion protein consisting of the yellow-green variant (referred to as enhanced yellow fluorescent protein; EYFP) of the Aequorea victoria green fluorescent protein (GFP) coupled to the mitochondrial targeting nucleotide sequence from subunit VIII of human cytochrome C oxidase. Upon transcription and translation of the plasmid in transfected mammalian hosts, the mitochondrial localization signal is responsible for transport and distribution of the fluorescent protein chimera throughout the cellular mitochondrial network. Tubular mitochondria can be subsequently visualized using fluorescence microscopy. The semi-confluent culture of MDCK cells illustrated above was first transiently transfected with a plasmid chimera containing the coding sequence for enhanced yellow fluorescent protein fused to the mitochondrial targeting sequence from subunit VIII of human cytochrome C oxidase. After fixation and permeabilization, the cells were counterstained for filamentous actin with Alexa Fluor 546 conjugated to phalloidin and for DNA with the nuclear-specific dye, DAPI. Images were recorded in grayscale with a QImaging Retiga Fast-EXi camera system coupled to an Olympus BX-51 microscope equipped with bandpass emission fluorescence filter optical blocks provided by Omega Optical. During the processing stage, individual image channels were pseudocolored with RGB values corresponding to each of the fluorophore emission spectral profiles. |
© 1995-2025 by Michael W. Davidson and The Florida State University. All Rights Reserved. No images, graphics, software, scripts, or applets may be reproduced or used in any manner without permission from the copyright holders. Use of this website means you agree to all of the Legal Terms and Conditions set forth by the owners.
This website is maintained by our
|