Fluorescence Digital Image Gallery
Madin-Darby Canine Kidney Epithelial Cells (MDCK)
|
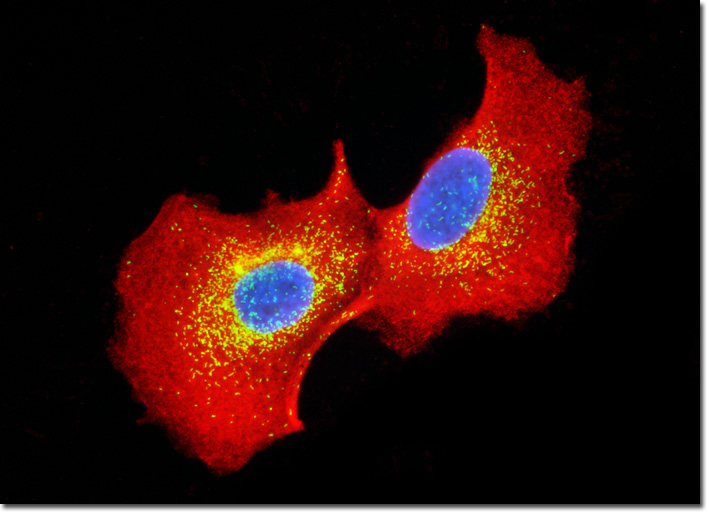
Clathrin is a protein centrally involved in the formation of the membrane-bound cellular packages known as vesicles. Composed of three heavy chains and three light chains, clathrin exists in the shape of a triskelion, a structure with three bent legs radiating from a central point. During vesicle formation, clathrin complexes are found assembled into polyhedral lattices, often referred to as clathrin baskets. The precise mechanisms involved in the formation of clathrin baskets are not yet known, nor are the exact mechanisms associated with the removal of the lattice, though both areas are currently being intensely researched. Studies have convincingly shown, however, that clathrin coats are involved in the process of protein sorting and that an enzyme known as uncoating ATPase is central to removing the lattices from vesicles. The culture of MDCK cells presented above was immunofluorescently labeled with primary anti-clathrin (heavy chain) mouse monoclonal antibodies followed by goat anti-mouse Fab fragments conjugated to the cyanine dye Cy3 in order to target the cytoskeletal network. In addition, peroxisomes present in the culture were immunofluorescently labeled with Cy2 conjugated to goat secondary antibodies directed against rabbit anti-PMP 70 (peroxisomal membrane protein 70) primary antibodies. Nuclei were counterstained with Hoechst 33342. Images were recorded in grayscale with a QImaging Retiga Fast-EXi camera system coupled to an Olympus BX-51 microscope equipped with bandpass emission fluorescence filter optical blocks provided by Omega Optical. During the processing stage, individual image channels were pseudocolored with RGB values corresponding to each of the fluorophore emission spectral profiles. |
© 1995-2025 by Michael W. Davidson and The Florida State University. All Rights Reserved. No images, graphics, software, scripts, or applets may be reproduced or used in any manner without permission from the copyright holders. Use of this website means you agree to all of the Legal Terms and Conditions set forth by the owners.
This website is maintained by our
|