Fluorescence Digital Image Gallery
Mongoose Skin Fibroblast Cells (APM)
|
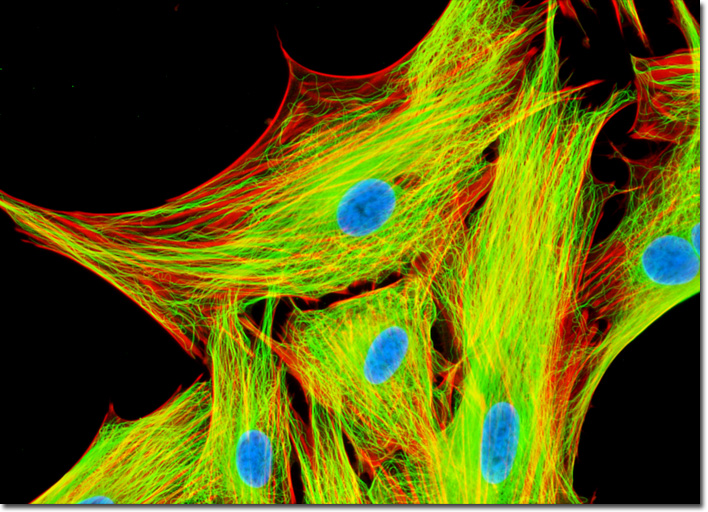
Each of the 13 protafilaments that comprise a microtubule consists of alpha-tubulin and beta-tubulin molecules organized in an alternating pattern. Upon coming together to fashion a microtubule, new bonds are formed amid the tubulin heterodimers. Between the alpha-tubulin molecules and adjacent beta-tubulin molecules along the longitudinal axis, interfaces with considerable binding energy are created. In addition, lateral interfaces arise between protafilaments that flank one another, primarily creating bonds between tubulin monomers of the same variety because of the manner in which the protafilaments align with one another (alpha-alpha, beta-beta). Due to the multiple interfaces that each subunit in a microtubule is subject to, the components are held quite stably in place. Therefore, if any subunits are added or subtracted from the structure, the event almost always involves an end of the filament, where molecules experience a fewer number of contacts. After fixation in 0.3-percent glutaraldehyde and permeabilization with Triton X-100, the adherent culture of APM cells presented above was treated with mouse anti-alpha-tubulin monoclonal primary antibodies followed by goat anti-mouse secondary antibodies (IgG) conjugated to Oregon Green 488. Mixed together with the secondary antibody was a phalloidin conjugate of Alexa Fluor 594, targeting the filamentous actin network. Nuclei were visualized by staining with Hoechst 33258 in Hanks' buffer for 30 minutes. Images were recorded in grayscale with a QImaging Retiga Fast-EXi camera system coupled to an Olympus BX-51 microscope equipped with bandpass emission fluorescence filter optical blocks provided by Omega Optical. During the processing stage, individual image channels were pseudocolored with RGB values corresponding to each of the fluorophore emission spectral profiles. |
© 1995-2025 by Michael W. Davidson and The Florida State University. All Rights Reserved. No images, graphics, software, scripts, or applets may be reproduced or used in any manner without permission from the copyright holders. Use of this website means you agree to all of the Legal Terms and Conditions set forth by the owners.
This website is maintained by our
|