Hoffman Modulation Contrast
The Hoffman Modulation Contrast system is designed to increase visibility and contrast in unstained and living material by detecting optical gradients (or slopes) and converting them into variations of light intensity. This technique was invented by Dr. Robert Hoffman in 1975, and employs several accessories that have been adapted to a number of commercial microscopes.
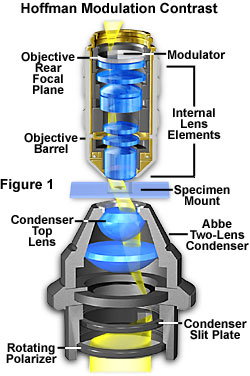
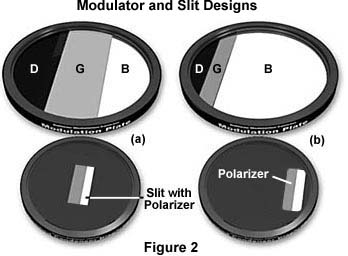
The basic microscope configuration for Hoffman Modulation Contrast is illustrated in Figure 1. An optical amplitude spatial filter, termed a "modulator" by Hoffman, is inserted on the back focal plane of an achromat or planachromat objective (although higher correction can also be used). Light intensity passing through this system varies above and below an average value, which by definition, is then said to be modulated. Objectives useful for modulation contrast can cover the entire magnification range of 10x to 100x. The modulator has three zones that are depicted in Figure 2: a small, dark zone near the periphery of the back focal plane which transmits only one percent of light (areas marked "D" in Figure 2); a narrow gray zone which transmits 15 percent (areas marked "G" in Figure 2); and the remaining clear or transparent zone, covering most of the territory at the back of the objective, which transmits 100 percent of the light (areas marked "B" in Figure 2). Unlike the phase plate in phase contrast microscopy, the Hoffman modulator is designed not to alter the phase of light passing through any of the zones. When viewed under modulation contrast optics, transparent objects that are essentially invisible in ordinary brightfield microscopy take on an apparent three-dimensional appearance dictated by phase gradients. The modulator does not introduce changes in the phase relationship of light passing through different portions of the modulator, but influences the principal zeroth order maxima. Higher order diffraction maxima are unaffected. Measurements using a Michelson interferometer confirm that the phase changes of light passed through a Hoffman-style modulator varies (if any) by a factor of less than l/20.
Below the stage, a condenser with rotating turret is utilized to hold the remaining components of the Hoffman Modulation Contrast system. The turret condenser has a brightfield opening with an aperture iris diaphragm for regular brightfield microscopy and for alignment and establishing proper conditions of Köhler illumination for the microscope. At each of the other turret openings, there is an off-center slit that is partially covered with a small rectangular polarizer. The size of the slit/polarizer combination is different for each objective of different magnification; hence the need for a turret arrangement.
The Hoffman design is such that the slits are located at the front focal plane of the condenser as illustrated in Figures 1 and 3. When light passes through the off-axis slit, it is imaged at the back focal plane of the objective (also termed the Fourier plane) where the modulator has been installed. The front focal plane of the condenser containing the off-axis slit plate is optically conjugate to the modulator in objective back focal plane. Image intensity is proportional to the first derivative of the optical density in the specimen, and is controlled by the zeroth order of the phase gradient diffraction pattern.
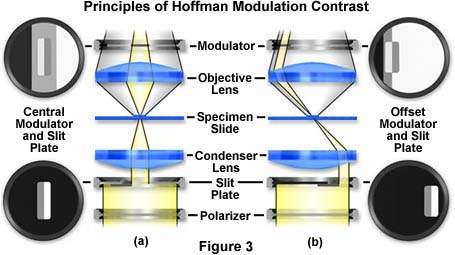
The principles of modulation contrast provide for at least two basic modulator-slit plate configurations that are illustrated in Figures 2 and 3. Drawings of the modulator plates shown in Figure 2 are exaggerated and increased in size for the purposes of this discussion. The arrangement on the left in Figures 2 and 3 (Figure 2(a) and Figure 3(a)) is a symmetrical system where both the modulator gray stripe and the slit are placed in the optical axis (center) of the microscope. Resolution in this system is limited to:
where NA is the numerical aperture of the objective and l equals the wavelength average of the imaging light source. The dark (one percent transmittance) and light or transparent (100 percent transmittance) zones are identical in size, while the gray (15 percent transmittance) zone is in the form of a narrow stripe that is 10 percent of the diameter of the exit pupil of the objective. The other arrangement (Figures 2 (b) and 3(b)) is asymmetrical or offset, where the dark area of the modulator lies outside the exit pupil of the objective. The resolution in this system is much improved and approaches:
where values for NA and l are the same as described above. It is obvious that resolution in the offset system (Figure 3(b)) is almost twice as good as in the central (Figure 3(a)) system. The transparent (clear) zone in the offset system fills almost 90 percent of the diameter of the objective exit pupil with the gray and dark zones filling the other 10 percent.
Below the condenser, a circular polarizer is placed on the light exit port of the microscope (note that both polarizers are below the specimen). The rotation of this polarizer can control the effective width of the slit opening. For example, a "crossing" of both polarizers at 90 degrees to each other results in "narrowing" the slit so that its image falls within the gray area of the modulator, as illustrated in Figure 3. The part of the slit controlled by the polarizer registers on the bright area of the modulator. As the polarizer is rotated, contrast can be varied for best effect. A very narrow slit produces images that are very high in contrast with a moderate degree of coherence. Optical section imaging is also optimized when the slit is adjusted to its narrowest position. When the circular polarizer is oriented with its vibration direction parallel to that of the polarizer in the slit, the effective slit width is at a maximum. This reduces overall image contrast and coherence, but yields much better images of thicker objects where large differences in refractive index exist.
Early designs of the modulation contrast system did not utilize the slit polarizer or the circular polarizer on the microscope light port, and relied on a single-sized slit as illustrated in Figure 4 for a symmetrical configuration. In this figure, light from the source passes through a fixed-aperture slit (termed a "slit-plate" in the figure) and then through a specimen containing phase gradients. These gradients deflect light, according to the direction of the gradient, into either the clear or dark zones of the symmetrical modulator that is positioned in the rear focal plane of the objective. The resulting image displays a simple contrast gradient that is dictated by the positioning and slope of phase gradients in the sample.
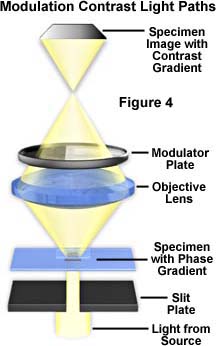
In modern advanced modulation contrast systems, both the modulator and the slit are offset from the optical axis of the microscope. This arrangement permits fuller use of the numerical aperture of the objective and results in good resolution and details. Shapes and details are rendered in shadowed, pseudo three-dimensional appearance. These appear brighter on one side, gray in the central portion, and darker on the other side, against a gray background. The modulator converts optical phase gradients in details (steepness, slope, rate of change in refractive index, or thickness in specimen details) into changes in intensity of various areas of the image at the plane of the eyepiece diaphragm. Resulting images have an apparent three-dimensional appearance with directional sensitivity to optical gradients.
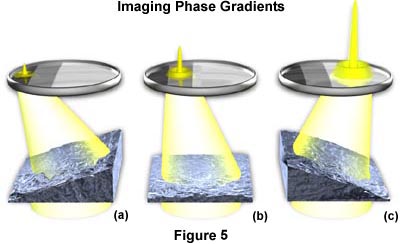
Opposite gradients result in deflection of the slit image to either the very dark part of the modulator or the bright section of the modulator, as illustrated in Figure 5. In this figure, a hypothetical specimen containing both positive and negative phase (thickness) gradients and a flat (non-gradient) area is imaged using modulation contrast optical components. The negative gradient depicted in Figure 5(a) deflects light into the dark area of the modulator, where it is attenuated to approximately one percent of its former value. Likewise in Figure 5(c), light deflected by a positive gradient into the clear area of the modulator is not attenuated, and 100 percent of this light is transmitted into the intermediate image plane. Any non-gradient part of the specimen (Figure 5(b)) and also the background (surround) register on the gray part of the modulator, where about 15 percent of the light is transmitted into the intermediate image plane. The result is that the intensity of the image area from one side of the gradient is dark. Intensity from the opposite side of the gradient yields a bright image area, and the non-gradient areas appear gray on the image, as does the background.
The contrast (related to variations in intensity) of the dark and bright areas against the gray gives a shadowed pseudo-relief effect. This is typical of modulation contrast imaging. Rotation of the polarizer alters the contrast achieved and the orientation of the specimen on the stage (with respect to the polarizer and offset slit) may dramatically improve or degrade contrast.
Since the modulator affects the image of the slit according to how the specimen's details shift the image of the slit (and thus results in altering light intensities), it is described as an amplitude filter. Hoffman and others have demonstrated that phase gradients in the specimen, like spatial frequencies, are distributed over the entire exit pupil of the objective. The optical transmission intensity distribution of the modulator will provide satisfactory images of a wide variety of objects that produce phase gradients including: all types of cells and tissues (both live, stained, and unstained), and surface details of crystals, transparent polymers, glasses and other similar materials. Reflected light modulation contrast microscopy is also useful for imaging grain boundaries in opaque and metallurgical specimens and surface details of complex integrated circuits and other electronic materials.
There are numerous advantages as well as limitations to modulation contrast. Some of the advantages include fuller use of the numerical aperture of the objective yielding excellent resolution of details along with good specimen contrast and visibility. Although many standard modulation contrast objectives are achromats or planachromats, it is also possible to use objectives with a higher degree of correction for optical aberration, as discussed above. Many major microscope manufacturers now offer modulation contrast objectives in fluorite-correction grades, and apochromats can be obtained by special order. Older objectives can often be retrofitted with a modulator made by Modulation Optics, Inc., the company founded by Dr. Robert Hoffman specifically to build aftermarket and custom systems.
In addition to the advantages of using higher numerical apertures with modulation contrast, it is also possible to do "optical sectioning" with this technique. Sectioning allows the microscopist to focus on a single thin plane of the specimen without interference from confusing images arising in areas above or below the plane that is being focused on. The depth of a specimen is measured in a direction parallel to the optical axis of the microscope. Focusing the image establishes the correct specimen-to-image distance, allowing interference of the diffracted waves to occur at a pre-determined plane (the image plane) positioned at a fixed distance from the eyepiece. This enables diffracting objects that occur at different depth levels in the specimen to be viewed separately, provided there is sufficient contrast. The entire depth of a specimen can be optically sectioned by sequentially focusing on each succeeding plane. In this system, depth of field is defined as the distance from one level to the next where imaging of distinct detail occurs, and is controlled by the numerical aperture of the objective. Higher numerical aperture objectives exhibit very shallow depths of field and the opposite holds for objectives of lower numerical aperture. The overall capability of an objective to isolate and focus on a specific optical section diminishes as the optical homogeneity of the specimen decreases.
Images appear shadowed or pseudo three-dimensional, enhancing visibility because of differences in contrast on either side of a detail. There are no halos exhibited in the image, unlike the images produced with phase contrast optics. Modulation contrast converts phase gradient information into amplitude differences that are very different from the phase relationship variations (and optical path differences) produced by a phase contrast microscope. Use of the dark and gray zones in the modulator produce images that contain varying shades of gray and are devoid of color. It is possible to introduce color into modulation contrast images by producing modulators that have the gray and dark zones substituted for colored zones of equal transmittance values. In this case, resulting images from the phase gradient are rendered in colors with similar gradients having the same tint. Currently, we are unaware of any commercial source of modulation filters that contain colored zones.
| Interactive Tutorial | |||||||||||
|
|||||||||||
Achromats or planachromats are the most widely utilized objectives for modulation contrast microscopy because they can yield good images since color is not involved. Using these objectives with a green filter (placed under the polarizer) will further improve the image because achromats are spherically corrected for green light. Objectives of higher correction, including fluorites and apochromats, can also be used for modulation contrast microscopy, but the added expense is often not worth the improvement in image quality, except at very high magnifications.
The cost of the modulation contrast accessories is considerably below that of differential interference contrast (DIC) equipment. Although both techniques require turret condensers with components matched to each objective, DIC-equipped microscopes also contain a polarizer below the condenser and an analyzer that is placed before the intermediate image plane in the optical path (above the objectives). The presence of a crossed polarization system, necessary for DIC microscopy, diminishes its effectiveness with samples that react to polarized light.
Birefringent objects (rock thin sections, crystals, bone, etc.), that can confuse images in DIC, can be examined since the specimen is not situated between the two polarizers. Further, specimens can be contained in plastic or glass vessels without deterioration of the image because of polarization effects since such vessels are also above both polarizers, not between them. This allows the Hoffman system to be far more useful than DIC in the examination and photomicrography of cell, tissue, and organ culture performed in plastic containers.
When the condenser is set at the brightfield position, objectives with a modulator installed can also be used for regular brightfield work. Because the modulator is off-axis, little deterioration of the image results. Objectives (but not the slit-plate condenser) equipped with a modulator can also be used for fluorescence and darkfield work, but these objectives should be avoided when attempting DIC microscopy. The modulation contrast system has been used very successfully with polarized light microscopy to enhance the detection of both optical gradients and birefringency in the specimen. In this application, a non-polarized slit is used and the polarized light configuration should be parallel polarizers (although crossed polarizers will also produce good results, albeit with diminished illumination). An objective modified for modulation contrast is very useful using ordinary polarized illumination without a slit plate in the condenser.
There are also several disadvantages and limitations of the Hoffman Modulation Contrast system. Images must be viewed with caution because different observers can "see" a "hill" in the image as a "valley" or vice versa as the pseudo three-dimensional image is observed through the eyepiece. The system is most sensitive to gradients perpendicular to the length of the slit, resulting in the requirement for some degree of skill in orientation of the specimen for best effect.
The cost of modification of each objective and the condenser openings must be added to the basic cost of these accessories themselves. Complex, high numerical aperture, multi-element objectives are difficult or too expensive to modify. In recent years, Robert Hoffman's company, Modulation Optics of Greenvale, New York (a wholly owned subsidiary of Slant Fin Corporation), has been producing modified objectives and condensers. Modulation Optics specializes in modifying objectives produced by the leading microscope manufacturers. Some objectives are easily modified, while some are difficult or impossible to modify for the modulation optics specification. However, any objective can be used with one of the company's intermediate tube systems, including a broad range that spans macro camera lenses to 100x microscope objectives. Also included in this category are objectives designed to image specimens either dry or with immersion media (oil, water, and glycerin), single and multiple wavelength objectives, reflected and transmitted light objectives, and objectives corrected for either infinity or a finite tube length. Modulation Optics designs and manufactures their own condenser systems to satisfy a wide range of numerical aperture and working distance combinations.
Non-absorbing specimens are not rendered in color, with the exception of those observed using a special modulator containing semi-transparent colored filters in place of the gray and dark zones. Specimens that naturally absorb specific wavelengths or lightly stained are rendered in color as well as those observed with the combination of modulation contrast and fluorescence or with the combination of modulation contrast and polarized light.
Configuration of a microscope for Hoffman Modulation Contrast is straight-forward and basic steps are outlined below:
Hoffman Modulation Contrast in Transmitted Light
- Attach the pertinent modulation contrast-enabled objectives to the nosepiece of the microscope, and install the turret condenser containing the appropriate slit plates. If the microscope is equipped for differential interference contrast (DIC) or polarizing microscopy, remove all polarizers, retardation plates, and Wollaston or Nomarski prisms from the optical path.
- Place a stained specimen (preferably a tissue thin section) on the stage and, using the 10x objective (with a modulator installed), align the microscope for proper Köhler illumination, outlined in our section on Anatomy of the Microscope. The modulation contrast slit plate should be removed from the condenser for this operation. If the turret condenser has a position for brightfield illumination with an aperture diaphragm, rotate the turret to select this condenser.
- View the modulator plate in the back focal plane of the objective, using a Bertrand lens (common on polarized light microscopes), a phase telescope, or by simply removing an eyepiece and peering down the eye tube. Make certain that the sample is removed from the optical path or that it is moved to a clear area on the microscope slide.
- Select the slit aperture plate that corresponds to the 10x objective by moving the appropriate condenser (from the turret) into the optical path. There should be a set of adjustment screws or a lever that allows rotation and translation of the illuminating slit plate within the condenser.
- Place the circular polarizing filter on the microscope light port beneath the condenser. Rotate this filter while observing the slit image through the Bertrand lens (or phase telescope) and observe that the angle of rotation influences the amount of light (brightness) passing through the polarizer portion of the slit.
- Translate the image of the slit so that the opened portion lacking a polarizer is superimposed over the gray region of the modulator plate as illustrated in Figure 3(b). The portion of the slit containing the polarizing material should be imaged in the clear portion of the modulator just to the right of the gray region. Rotate the circular polarizing filter and observe how the region of the slit containing the polarizing material appears and disappears. When the vibration plane of the circular polarizer is aligned perpendicular to the vibration plane of polarizer in the slit, the slit size if minimized and maximum contrast is obtained. This maneuver can be practiced using the interactive tutorial below:
| Interactive Tutorial | |||||||||||
|
|||||||||||
- Remove the Bertrand lens or phase telescope and replace the eyepiece in the eye tube of the microscope. Place a specimen candidate on the stage and focus with the 10x objective.
- Readjust the condenser position by refocusing the field diaphragm to achieve a sharp focus. Open the field iris diaphragm until it is just outside the field of view.
- The image is now ready for observation or photomicrography with modulation contrast. Rotate the specimen and/or the circular polarizer at the base of the microscope to achieve optimum contrast. These settings will vary from specimen to specimen.
- Repeat the above steps each time a different magnification is selected for viewing the specimen in modulation contrast.
Once again, we find that manipulation of light at the front focal plane of the condenser (by means of an offset slit) and manipulation of light at the back focal plane of the objective (offset modulator) can have a significant effect upon the image presented in the eyepieces.
For more information about commercially available optics for Hoffman Modulation Contrast contact:
100 Forest Drive
Greenvale, NY 11542
(516)-484-8882
email: Modulation Optics
Contributing Authors
Mortimer Abramowitz - Olympus America, Inc., Two Corporate Center Drive., Melville, New York, 11747.
Michael W. Davidson - National High Magnetic Field Laboratory, 1800 East Paul Dirac Dr., The Florida State University, Tallahassee, Florida, 32310.
BACK HOFFMAN MODULATION CONTRAST
