Differential Interference Contrast
Comparison of Phase Contrast and DIC Microscopy
Phase contrast and differential interference contrast (DIC) microscopy are complementary techniques capable of producing high contrast images of transparent biological phases that do not ordinarily affect the amplitude of visible light waves passing though the specimen. Because phase differences are undetectable to the human eye, and are not readily observed in a microscope under brightfield illumination, the light path through the microscope must be suitably modified in order to produce satisfactory images of phase specimens. Many popular contrast-enhancing techniques rely on the ability of phase specimens to modify the optical path difference between waves traversing the biological material and those passing through the surrounding medium.
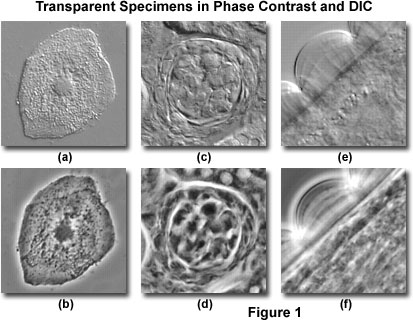
Perhaps the most fundamental distinction between differential interference contrast and phase contrast microscopy is the optical basis upon which images are formed by the complementary techniques. Phase contrast yields image intensity values as a function of specimen optical path length magnitude, with very dense regions (those having large path lengths) appearing darker than the background. Alternatively, specimen features that have relatively low thickness, or a refractive index less than the surrounding medium, are rendered much lighter when superimposed on the medium gray background.
The situation is quite distinct for differential interference contrast, where optical path length gradients (in effect, the rate of change in the direction of wavefront shear) are primarily responsible for introducing contrast into specimen images. Steep gradients in path length generate excellent contrast, and images display a pseudo three-dimensional relief shading that is characteristic of the DIC technique. Regions having very shallow optical path slopes, such as those observed in extended, flat specimens, produce insignificant contrast and often appear in the image at the same intensity level as the background.
Aside from the differences in contrast-formation mechanisms, DIC and phase contrast images differ in a number of other features. Illustrated in Figure 1 are several digital images comparing specimens captured in DIC and phase contrast. A human buccal mucosa epithelial (cheek) cell, revealing the nucleus, cytoplasmic inclusions, and numerous bacteria on the upper surface, is presented in Figure 1(a), imaged with differential interference contrast. The same viewfield with phase contrast illumination is illustrated in Figure 1(b). The phase contrast image features pronounced halos around the cellular periphery and nucleus, which are absent in the differential interference contrast image. Optical sectioning DIC microscopy investigations (not illustrated) reveal that the bacteria are present (almost exclusively) on the membrane surface that is bathed in surrounding media as opposed to lying on the underside of the cell. This fact cannot be unambiguously determined with phase contrast.
In Figure 1(c), a thick section of murine kidney tissue imaged with differential interference contrast reveals a bundle of cells enclosed within a tubule. A phase contrast image (Figure 1(d)) of the same area is confusing and disturbed by the presence of phase halos outside the plane of focus. However, several of the cellular nuclei appear visible in the phase contrast image, which are not distinguishable in DIC. Relatively high magnification views of an Obelia polypoid annulated stem perisarc in DIC and phase contrast are illustrated in Figures 1(e) and 1(f), respectively. In DIC, (Figure 1(e)) the annular structure appears hemispherical with internal rays that emanate radially from the stem. In addition, granular particles are visible within the stem structure, but anatomical detail is largely undefined. The phase contrast image (Figure 1(f)) is confused by halos around the annular rings and within the stem.
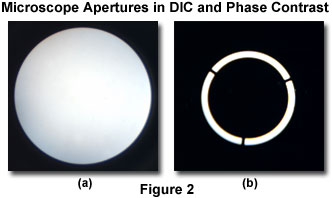
A primary advantage of differential interference contrast over phase contrast is the ability to utilize the instrument at full numerical aperture without the masking effects of phase plates or condenser annuli, which severely restrict the size of condenser and objective apertures. The major benefit is improved axial resolution, particularly with respect to the ability of the DIC microscope to produce excellent high-resolution images at large aperture sizes. Illustrated in Figure 2 are digital images acquired with a Bertrand lens of the objective rear aperture in differential interference contrast (Figure 2(a)) and phase contrast (Figure 2(b)) microscopes. In both cases, the objective is a 40x fluorite having a numerical aperture of 0.75, and the condenser aperture diaphragm is opened to the largest diameter size. Even with the polarizer in place and a Nomarski prism installed in the condenser, the DIC aperture is much larger than the phase contrast aperture, primarily because the phase condenser annulus restricts a significant portion of the illumination that would ordinarily traverse the microscope optical train.
Orientation Effects
Phase contrast lacks the pronounced azimuthal effect inherent in differential interference contrast, which is manifested by asymmetrical orientation of the beamsplitting Nomarski (or modified Wollaston) prisms with respect to the microscope optical axis and polarizers. The absence of orientation effect occurs because the annular phase plate in a phase contrast microscope condenser (Figure 2(b)) is rotationally symmetric through 360 degrees and illuminates the specimen evenly from all angles. As a result, the phase contrast image is independent of orientation and not subject to rotational-dependent contrast effects. For many specimens imaged in differential interference contrast, a prerequisite orientation in order to achieve maximum contrast (either parallel or perpendicular to the shear axis) restricts freedom of specimen rotation, especially for linear or closely-spaced periodic structures.
Azimuth contrast effects in differential interference contrast can be utilized to advantage by equipping the microscope with a 360-degree rotating circular specimen stage. Originally designed for observations in polarized light microscopy, circular stages enable the operator to rotate the specimen with respect to the prism shear axis in order to maximize or minimize contrast effects for selected specimen features. Contrast in DIC microscopy achieves a minimum level for linear phase specimens that extend along the direction of shear, but can be varied significantly by rotating the stage by a few degrees. Non-linear specimens, such as cells, tissue sections, particles, and amorphous polymers, do not display a significant azimuth effect in DIC, and can usually be satisfactorily imaged in a variety of orientations.
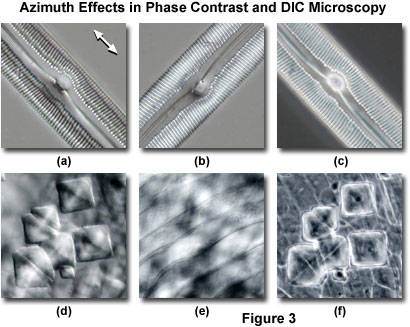
Presented in Figure 3 are two linear specimens having a significant amount of periodicity and asymmetry imaged in both phase contrast and DIC. In both cases, contrast in the images obtained from DIC is largely dependent upon the orientation of the specimen with respect to the shear axis of the microscope, while the phase contrast image features are independent of specimen rotation around the microscope optical axis. The images in Figures 3(a) and 3(b) were obtained in DIC from a mounted specimen of a bilaterally symmetrical elongated pennate diatom frustule. When the long axis of the diatom is aligned parallel to the Nomarski prism shear axis (Figure 3(a)) ridges and pores in the frustule are readily observed. The white arrow in the upper right-hand corner of Figure 3(a) denotes the Nomarski prism shear axis for the DIC images illustrated in Figure 3. Rotating the diatom by 90 degrees (perpendicular to the shear axis) reduces contrast in the frustule image and obscures important details present in the microstructure. For example, the longitudinal ridges readily observed at the edges and center of the frustule in Figure 3(a) are absent in Figure 3(b). Azimuth effects are absent in the same specimen when imaged using a phase contrast optical system (Figure 3(c)), which displays similar contrast regardless of the specimen orientation. Note that the image contrast in Figure 3(c) more strongly resembles that displayed by the DIC image in Figure 3(b) rather than the high contrast image presented in Figure 3(a). In this case, the benefits of azimuthal effects resulting from specimen orientation in differential interference contrast are clearly evident.
A second example of azimuth effects that are present in DIC, but lacking in phase contrast, is illustrated in Figures 3(d) through 3(f). The specimen is a semi-transparent ctenoid fish scale containing diamond-shaped inclusions that severely lack contrast and are difficult to distinguish when superimposed over the striated body spines of the scale. When the ctenoid scale is oriented with the body spines parallel to the shear axis in a DIC microscope (Figure 3(d)), the transparent rectangular inclusions become visible and mask the body spines. Rotating the microscope stage by 90 degrees reduces the contrast level of the inclusions, and the striated spines become clearly visible (Figure 3(e)). In phase contrast (Figure 3(f)), the inclusions are present with accompanying halos, as well as the striated spines, irrespective of the specimen orientation.
The correlation between image contrast and specimen orientation in differential interference contrast can often be used to advantage in the investigation of extended linear specimens. For example, the highly ordered structures in diatom frustules and similar specimens can overlap, leading to confusing images when observed in phase contrast and similar contrast-enhancing techniques. By capturing images at several orientations, DIC microscopy is often able to present a more clear understanding of the complex morphology present in many extended, linear specimens. In addition, when optical sectioning methodology is coupled to azimuth-specific imaging, differential interference contrast microscopy can often reveal features that are difficult, or impossible, to distinguish using alternative techniques.
Halo and Shade-Off Artifacts
The halo and shade-off artifacts that plague the phase contrast optical system are largely absent in differential interference contrast images. In positive phase contrast, specimen features with a higher refractive index than the surrounding medium are encircled with a bright fringe (halo) that often obscures edge detail. Although halos can be suppressed to a limited degree by configurational modifications to the objective phase plate design, they cannot be eliminated entirely. In some cases, differential interference contrast images suffer from excessive brightness along edges having very steep optical gradients, but this effect usually occurs when bias retardation values are too low, and can be circumvented by proper adjustment of the Nomarski prism (or de SÚnarmont compensator polarizer) position.
The presence and severity of halos in phase contrast is dependent, in part, on the refractive index difference between the specimen and the surrounding medium. Often, phase contrast specimens contain a wide variety of structures that display wildly fluctuating refractive indices, which together yield complex images. Areas having a lower refractive index than the surrounding medium exhibit dark halos as opposed to the bright halos that can be observed surrounding higher refractive index features. Halos arise because of the configurational parameters of the phase contrast microscope optical system. A small portion of incident light deviated by the specimen passes through the objective phase plate due to the spherical nature of diffracted wavefronts. Smaller specimen features give rise to larger diffraction angles, but large extended structures can produce diffracted wavefronts having shallower angles that often fall within the objective aperture zone occupied by the phase plates.
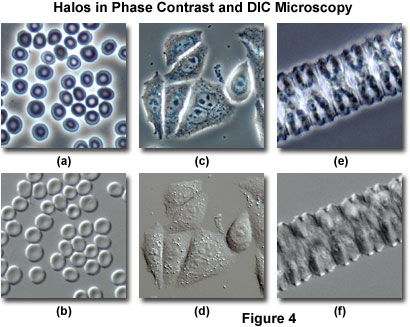
A comparison between phase contrast and DIC images with respect to halo artifacts is presented in Figure 4 for three commonly imaged transparent specimens. Human erythrocytes (red blood cells) exhibit a distinctive halo surrounding the disk-shaped cells when imaged in positive phase contrast at medium and high magnifications (Figure 4(a)). Note that the central portion of the erythrocyte disks is lighter than the periphery due to a reduced optical path length in this region. When the same viewfield is examined in differential interference contrast (Figure 4(b)), halo artifacts surrounding the cells are absent, but the images acquire a shadow-cast appearance oriented along the Nomarski prism shear axis (northwest to southeast), which is manifested by the upper left portion of the outer ridges being much lighter in appearance than the corresponding ridges on the opposite side (lower right) of the cells.
Living cells grown in monolayer culture are frequently observed and imaged using both phase contrast and differential interference contrast microscopy. Several adherent HeLa cells in culture on glass coverslips are illustrated in Figure 4(c) with phase contrast optics. Halo artifacts surround the periphery of the cell membranes and are also evident on some of the larger organelles within the cells. Although many of the internal cellular details are present at high resolution in this image, information about intracellular contracts is suppressed by the bright (halo) regions appearing between adjacent cell membranes. The absence of halo artifacts is evident in the same viewfield (Figure 4(d)) when imaged with differential interference contrast. Internal details of the cells are less evident in DIC, but the periphery of the cellular membranes is imaged more clearly than with phase contrast. In fact, the close proximity of neighboring cells is very obvious in the DIC image, which renders the technique more accurate for studying intercellular events, such as contact inhibition.
Members of the filamentous green algae genus, Zygnema, exhibit an elongated, linear structure composed of repeating individual cellular units. In phase contrast (Figure 4(e)), filamentous Zygnema specimens display a pronounced halo surrounding the exterior of the rod-like algae filaments, but also contain substantial internal detail that is obscured by the halo artifact. The bisymmetrical U-shaped repeating internal cellular structures (Figure 4(e)) give rise to large halos that significantly reduce contrast and mask smaller features. Differential interference contrast eliminates the halo artifacts present in phase contrast images of Zygnema (Figure 4(f)), and reveals substantially more of the internal detail present in the center of the filament. However, in order to obtain the most definitive conclusions regarding Zygnema filament structure, the two imaging techniques should be applied in conjunction (as is the case for many of the specimens described above).
As discussed above, specimen features having a higher refractive index than the surrounding medium display bright halos around the periphery and exhibit darker central portions when imaged with positive phase contrast optical systems. The opposite effect occurs when features having lower refractive index than the medium are observed (dark halos and light internal regions). Halo effects vary with the size of the specimen feature and are heavily dependent upon the refractive index differential between the specimen and the surrounding medium. Larger differences in refractive index tend to generate more pronounced halo effects, which are also dependent on instrumental parameters, such as phase plate dimensions and neutral density, and the objective magnification factor. In addition, specimen feature size plays a paramount role in determining the severity of halo artifacts in phase contrast microscopy.
The situation is quite different in DIC microscopy, where large refractive index differentials and specimen feature size do not impair image quality. In fact, pronounced differences of refractive index between the specimen and its surrounding medium are often highly desirable, and usually lead to excellent images in differential interference contrast. Feature size does not generally present a problem in DIC microscopy. Specimens having large fluctuations in size and refractive index can frequently be imaged simultaneously (side-by-side) with differential interference contrast optical systems to yield structures having excellent contrast, which enables even minute details to be observed and recognized.
Halo artifacts and image contrast are also affected by the optical thickness of the specimen to a much larger degree in phase contrast than in DIC microscopy. At large optical path differences (up to half a wavelength), ambiguous phase contrast images can be produced because the thickest features in a specimen often display contrast levels that are very close, or identical, to those of the thinnest features. This arises from the complex relationships between optical path length (specimen thickness and refractive index), contrast, and phase angles. In general, ideal phase contrast specimens should not have features exceeding about one-tenth wavelength in optical path difference. Thin specimens are also desirable for DIC microscopy, but the technique is capable of producing suitable images with specimens that have much larger thickness values (and optical path lengths) than are recommended for phase contrast. However, very thick specimens can negatively influence the overlap of interference planes between the objective and condenser prisms, reducing the quality and contrast in DIC images.
In flat, extended specimens of uniform optical path length, an artifact known as shade-off is commonly observed in phase contrast microscopy. Shade-off is manifested by the central portion of the specimen displaying a gradual decrease in contrast until the intensity approaches that of the surrounding medium. In extreme cases, extended specimens will only be visible in phase contrast due to the presence of their halos. In DIC, a variation in image intensity only occurs if there is a marked change in optical path difference, and the boundaries of extended specimens are often the only feature that can be recognized with this technique.
Depth of Field and Optical Sectioning
Because phase contrast images can become ambiguous (or even experience a reversal of contrast) when the specimen optical path length fluctuates over a large range, the technique should be restricted to specimens having a path difference of one-tenth wavelength or less, as previously discussed. In other words, thick specimens, or those having wedge-shaped structures, are generally not suitable for quantitative investigations with phase contrast microscopy. Thin specimens also tend to produce superior images in differential interference contrast, but thicker specimens (having an optical path difference between one-tenth and a full wavelength) can also be imaged at wide apertures using optical sectioning techniques.
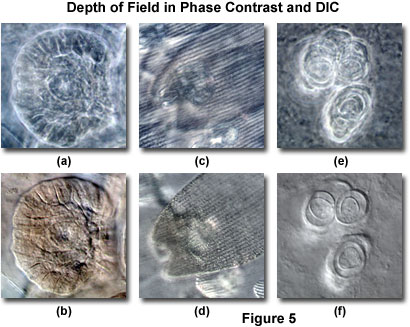
The illumination aperture in phase contrast is fixed by the size of the condenser annulus and cannot be varied to achieve increased or decreased depth of field. This restriction hampers observations of specimens containing significant halo artifacts, which can sometimes obscure focused specimen features due to overlapping details originating from excessive light originating outside the objective focal plane. Such problems often arise when attempting to image specimens that are too thick for phase contrast. However, as previously discussed, the optical sectioning capability of differential interference contrast microscopy (at wide condenser aperture sizes) enables excellent images to be obtained with relatively thick specimens.
The benefits of large aperture optical sections from thick specimens in differential interference contrast are illustrated in Figure 5, and compared to the same viewfields imaged with phase contrast. Figure 5(b) illustrates a medusa bud from a reproductive polyp of the Obelia hydrozoan imaged at high magnification in DIC with the condenser iris diaphragm opening set to approximately 95 percent of the objective aperture size. The resulting thin optical section reveals sharply focused image details of the medusa features, which are only minimally obscured by light originating away from the focal plane. When the same viewfield is examined in phase contrast (Figure 5(a)), the medusa image is blurred by halos and exhibits reduced resolution due to the restricted aperture of the optical system.
Similar situations are presented in Figures 5(c) through 5(f). Figure 5(c) depicts overlapping wing scales from the monarch butterfly (Danaus plexippus), one of the most common butterflies in North America. Individual scales are not distinguishable in Figure 5(c) because of halo artifacts and indistinct features that are not in the plane of focus. In general, the image is confused by numerous artifacts, making specimen features difficult to decipher. Imaging the same specimen in differential interference contrast (Figure 5(d)) eliminates many of the distracting artifacts positioned away from the focal plane and clearly delineates the surface features in a single wing scale. Likewise, an egg packet within the abdomen of a canine cucumber tapeworm (Dipylidium caninum) reveals sharp edges surrounding individual eggs when imaged in DIC (Figure 5(f)). However, the focused eggs are devoid of distinctive structure in phase contrast (Figure 5(e)), primarily due to halos from others eggs situated away from the focal plane.
The optical sectioning capability of differential interference contrast at large condenser aperture sizes and high numerical aperture is critical for imaging single focal planes in thick phase specimens. Details and stray light from features lying outside of the immediate focal plane do not compromise DIC images in the same manner as phase contrast images, which are often complicated by halo artifacts. As a result, differential interference contrast can be employed to obtain excellent images under somewhat unfavorable conditions (very thick specimens), even when large depth of field renders the identification of phase structures impossible in phase contrast microscopy due to overlapping details.
Birefringent Specimens
An advantage of phase contrast over DIC is the ability to successfully image dichroic and birefringent specimens. Because differential interference contrast optical systems rely on polarized light to establish the orientation of wavefront fields, differential absorption of ordinary and extraordinary waves by a birefringent or dichroic specimen leads to confusing images. Phase contrast does not require the use of polarized light, and is free of optical disturbances generated by birefringent specimens.
One of the primary concerns pertaining to birefringence artifacts in differential interference contrast microscopy arises from the widespread use of plastic tissue culture vessels. Stress inherent in the molded polymer vessel structure produces excessive birefringence that depolarizes light and confuses the interpretation of DIC images. Thus, living cells grown in these containers cannot be imaged satisfactorily with differential interference contrast and, instead, are usually observed and photographed with phase contrast or Hoffman modulation contrast optical systems.
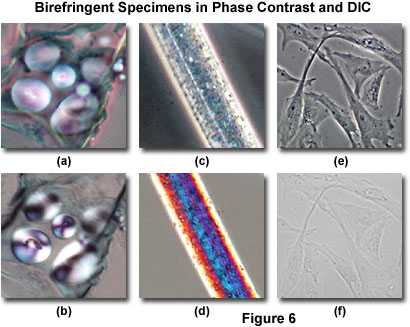
Typical artifacts arising from imaging birefringent specimens in DIC are presented in Figure 6, along with images of identical viewfields in phase contrast. Starch storage granules (appearing as globules) from a quadruple-stained thin section of potato (Solanum tuberosum) tissue, also illustrating a portion of the mature tuber with periderm, cortex, and reduced vascular tissues, are presented in Figures 6(a) and 6(b). Because carbohydrates in potato starch grains display an ordered lamellar molecular structure, portions of the grains (and in some cases, the entire grain itself) are birefringent and absorb the polarized wavefronts that leave the condenser Nomarski prism and pass through the specimen. As a result, the potato starch grains observed in DIC microscopy exhibit interference colors and the characteristic Maltese cross patterns (originating from the crossed polarizers), which are typical of birefringent anisotropic specimens having spherical symmetry (Figure 6(b)). The birefringence artifact in DIC masks much of the internal structure of the starch grains, which reveal far more detail when imaged in phase contrast (Figure 6(a)), even though the specimen has been stained.
Illustrated in Figure 6(c) is a phase contrast image of a single rayon fiber showing the pitted morphology that is present both on the surface and inside the polymer. Rayon is fabricated from cellulose, cotton linters, or wood chips by treating the bulk materials with sodium hydroxide and carbon disulfide, and then passing the resulting viscous solution through spinnerets. The fibers produced in this process have long-range order and are usually very highly birefringent. DIC examination (Figure 6(d)) of the rayon fiber confirms the birefringent character, which is manifested in Newtonian interference colors present in the image. The colors, while beautiful, obscure specimen detail and decrease the usefulness of DIC for imaging materials of this type. The birefringent images illustrated in Figures 6(b) and 6(d) were recorded using relatively thin (5-20 micrometer) specimens at low magnification (20x) that remain in sharp focus throughout the viewfield. When thicker birefringent specimens are imaged with DIC, the interference colors originating from planes away from the point of focus tend to obscure specimen detail to a much greater degree. Therefore, as specimen thickness increases (for birefringent specimens), the resulting images produced in DIC tend to lose contrast and the quality deteriorates rapidly.
The problems associated with observing living cells grown in plastic tissue culture vessels using differential interference contrast microscopy are clearly evident by comparing Figures 6(e) and 6(f). Several Indian Muntjac (Muntiacus muntjak) deer skin fibroblast cells in monolayer culture are illustrated in Figure 6(e), imaged using a long working distance objective and condenser combination in phase contrast on an inverted tissue culture microscope. The cells were grown in molded polystyrene vessels having an upper and lower wall thickness of approximately 1.2 millimeters each (combined thickness of polystyrene is 2.4 millimeters). Even with phase contrast objectives having correction collars that are designed for observation through thick glass or plastic, the image quality suffers when compared to cells imaged on thin (170 micrometer) cover glass plates (compare Figure 6(e) to Figure 4(c)). However, much of the internal cellular detail is still visible, including nuclei, the nucleolus, vacuoles, and numerous smaller particles.
In differential interference contrast, the cultured Indian Muntjac fibroblast cells illustrated in Figure 6(e) lose a significant amount of contrast (Figure 6(f)) due to absorption and interference effects from the birefringent polystyrene container. Because the container walls are relatively thick, strain birefringence depolarizes the ordinary and extraordinary wavefronts in the upper wall of the tissue culture vessel before they pass through the cells, and once again after exiting the cells and passing through the lower wall. The result is a drop in contrast so severe, the cells can be imaged only when the condenser diaphragm is almost completely closed (similar to brightfield techniques with transparent specimens). Under these conditions, images display numerous diffraction artifacts in addition to the low contrast, and render DIC practically useless for this type of observation.
The major source of errors in examining birefringent specimens with differential interference contrast microscopy arises from the necessity of using polarized light, as discussed above. In birefringent specimens, the polarized orthogonal (ordinary and extraordinary) wavefronts are absorbed to different degrees, depending upon their orientation with respect to anisotropic molecules within the specimen. As a result, when the orthogonal wavefronts are recombined in the objective Nomarski prism and pass through to the analyzer, they interfere with different intensity. The final image, therefore, is not only a function of the optical path difference experienced by the two wavefronts (normally a critical factor in DIC), but also the differential amplitudes of the two beams induced by the specimen. The effect on images is similar to a microscope configuration in which the transmission axes of the polarizer and analyzer are not perfectly perpendicular (in effect, slightly uncrossed). Because phase contrast does not rely on polarized light, the technique is largely free of artifacts induced by birefringent specimens.
Stained Specimens
Lightly stained amplitude specimens, which are not necessarily birefringent but may retain a significant amount of phase gradient character, experience reduced intensity due to absorption of visible wavelengths by the objective phase plate in phase contrast microscopy. However, these specimens can often produce satisfactory results in differential interference contrast when substantial optical path gradients are present and both polarized wavefronts are equally absorbed. In fact, selective staining of intracellular organelles (for instance, intact nuclei and metaphase chromosomes) can be coupled to imaging with DIC microscopy in order to enhance specimen detail and segregate events occurring at the time the cells were fixed.
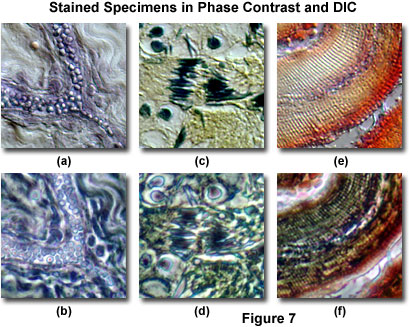
Illustrated in Figure 7 are several examples of specimens stained with common biological (visible light absorbing) chromophores, and imaged with both phase contrast and DIC microscopy. A thin section of human fat-stained adipose tissue observed in differential interference contrast is presented in Figure 7(a), and the same viewfield with phase contrast in Figure 7(b). The phase contrast image is confusing and lacks resolution of fine detail, primarily because of halo artifacts that mask the advantages of the biological stain. Phase contrast is clearly not appropriate for retrieving any significant information from this specimen. In distinction, a corresponding DIC image of the same specimen (Figure 7(a)) renders the fat globules in shadow-cast pseudo three-dimensional relief, which distinguishes them quite well from other features in the image.
The same effect is observed with DIC microscopy in Figure 7(c), where differentially stained meiotic nuclei and chromosomes within a frog testes thin section can be observed in high contrast. This image benefits from the clever application of multiple stains that aid in chromatically segregating the genetic material from other intracellular components. In fact, the specimen yields more significant information when imaged in DIC than it does in the target observation mode of brightfield illumination. The same viewfield in phase contrast (Figure 7(d)) is confused by halos surrounding the densely stained intracellular components, which are far more difficult to distinguish in this imaging mode. A similar situation exists for a mammalian tooth stained thin section presented in Figures 7(e) and 7(f). In DIC, striated lamella in the enamel are easy to observe due to their relief (Figure 7(e)), while less than optimal optical path differences coupled to overstaining mask these structures in phase contrast (Figure 7(f)). The examples in Figure 7 provide strong evidence that differential interference contrast is a suitable technique for observing lightly stained specimens, even though the same specimens are difficult to image in phase contrast.
Conclusions
The most obvious difference between DIC and phase contrast microscopy is the pseudo three-dimensional shadow-cast images formed by differential interference contrast optical systems. Specimen images are rendered in a manner reminiscent of the shadowing techniques utilized for many years in electron microscopy, which yield information on topographical properties of the specimen. However, in DIC, the apparent hills and valleys in images must not be mistaken as an indication of actual topographical profile in the specimen, and should always be interpreted with caution. Phase contrast does not produce images having significant three-dimensional character.
Many of the similarities and differences between phase contrast and differential interference contrast microscopy are summarized in Table 1 for a number of variables, including image brightness, resolution, phase shift limitations, artifacts, specimen characteristics, and cost. In summary, image brightness in both phase contrast and DIC is much less (only a few percent) than ordinary brightfield observation, but these contrast-enhancing techniques are capable of providing far more highly defined images. Illumination is more critical for DIC microscopy than phase contrast, especially when using low transmission polarizers at high magnifications. Often, a 100-watt tungsten-halogen or mercury arc-discharge lamp is required for satisfactory imaging, whereas phase contrast microscopes perform adequately with 50-watt incandescent lamps. The lateral (x-y) and axial (z) resolution in differential interference contrast microscopy dramatically exceeds that with phase contrast, which exhibits modest or poor resolution in both dimensions even though the phase shift detection limit is similar in both cases.
Characteristics of Phase Contrast and DIC Microscopy
|
|||||||||||||||||||||||||||||||||||||||||||||||
Table 1
Thick phase specimens having large optical path differences can be readily imaged in differential interference contrast using optical sectioning techniques, but are often difficult to image with phase contrast due to halo artifacts. Other factors, such as azimuthal effects and the ability to image stained specimens in DIC, help to offset the two techniques. Phase contrast is far more useful for routine observations in tissue culture laboratories, where imaging living cells in plastic culture vessels is a daily activity. Alternatively, high resolution information is best retrieved using differential interference contrast methods (often coupled to time-lapse video) for live cell imaging. In general, DIC requires more technical skills to configure and operate, including the complex alignment process, continuous manipulation of specimen focus, aperture iris diaphragm adjustment, specimen orientation, and lateral displacement of the objective Nomarski prism to achieve optimum contrast. Phase contrast microscopes are far easier to align and operate, and can be utilized quite effectively by relatively inexperienced microscopists.
Phase contrast is generally limited in utility to imaging transparent phase specimens having poor contrast. Conversely, DIC microscopy can be employed both for stained and unstained specimens, but suffers from artifacts when birefringent features are encountered. More control over specimen contrast is afforded by translating the objective prism (or de SÚnarmont compensator) in DIC, which can be adjusted over a wide range of positions to achieve varying levels of grayscale interference colors, or multi-colored optical staining effects at large path differences. In addition, the condenser aperture diaphragm can also be adjusted in DIC to alter specimen contrast and resolution. Phase contrast is limited to the contrast afforded by the condenser annular diaphragm and the objective phase plate housed in the objective. Virtually no adjustment of contrast (other than exchanging phase plates) is possible with phase contrast microscopy.
Among the most important considerations in many laboratories, when comparing DIC and phase contrast, is the cost of accessory components. Because DIC requires several expensive birefringent Nomarski prisms and strain-free optical elements (objectives and condenser), the instrumentation is significantly more expensive than phase contrast components. In many cases, particularly when photomicrography and/or digital imaging is not a primary consideration, phase contrast will often serve the purposes of a laboratory. However, when high-resolution images must be obtained from living cells and tissues, or similar fragile transparent phase specimens, differential interference contrast is the technique of choice.
In general, phase contrast and differential interference contrast microscopy should be considered as complementary (rather than competing) techniques, and employed together to fully investigate specimen optical properties, dynamics, and morphology. The introduction of modern phase contrast objectives having phase plates of lower neutral density enables the microscopist to, in many cases, utilize a single set of objectives for brightfield, fluorescence, phase contrast, and DIC imaging.
Contributing Authors
Douglas B. Murphy - Department of Cell Biology and Anatomy and Microscope Facility, Johns Hopkins University School of Medicine, 725 N. Wolfe Street, 107 WBSB, Baltimore, Maryland 21205.
Jan Hinsch - Leica Microsystems, Inc., 90 Boroline Road, Allendale, New Jersey, 07401.
Kenneth R. Spring - Scientific Consultant, Lusby, Maryland, 20657.
Michael W. Davidson - National High Magnetic Field Laboratory, 1800 East Paul Dirac Dr., The Florida State University, Tallahassee, Florida, 32310.
BACK TO DIFFERENTIAL INTERFERENCE CONTRAST MICROSCOPY
BACK TO PHASE CONTRAST MICROSCOPY
