Sources of Visible Light
Visible light comprises only a tiny fraction of the entire electromagnetic radiation spectrum, yet it contains the only region of frequencies to which the rods and cones of the human eye will respond. The wavelengths that humans are typically able to visualize lie in a very narrow range between approximately 400 and 700 nanometers. Humans can observe and respond to stimuli created by visible light because the eyes contain specialized nerve endings that are sensitive to this range of frequencies. However, the remainder of the electromagnetic spectrum is invisible.
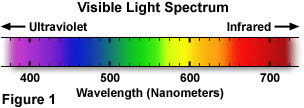
A wide variety of sources are responsible for emission of electromagnetic radiation, and are generally categorized according to the specific spectrum of wavelengths generated by the source. Relatively long radio waves are produced by electrical current flowing through huge broadcast antennas, while much shorter visible light waves are produced by the energy state fluctuations of negatively charged electrons within atoms. The shortest form of electromagnetic radiation, gamma waves, results from decay of nuclear components at the center of the atom. The visible light that humans are able to see (the spectrum is illustrated in Figure 1) is usually a mixture of wavelengths whose varying composition is a function of the light source.
In our everyday lives, we are bombarded by an enormous spectrum of electromagnetic radiation, only a portion of which we are able to actually "see" as visible light. When venturing outside, a vast majority of the light visible to humans is emitted from the sun, which also produces many other frequencies of radiation that do not fall into the visible range. Inside, we are exposed to visible light that originates from artificial sources, primarily fluorescent and incandescent tungsten devices.
At night, natural light is produced by celestial bodies, such as the moon, planets, and stars, in addition to the periodic Aurora Borealis (Northern Lights), and the occasional comet or meteor ("shooting star"). Other natural light sources include meteorological lightning, volcanoes, forest fires, plus some biochemical sources of visible light (bioluminescence). The biological light sources include the familiar lightning bugs ("fireflies") and more exotic glows from the sea, including bioluminescent species of bacteria, algae, dinoflagellates, jellyfish, comb-jellies (ctenophores), and some species of fish.
Visible Light Wavelength and Perceived Color
|
||||||||||||||||||||
Table 1
Table 1 contains a listing of the apparent color distribution perceived by humans for a number of narrow wavelength bands in the visible light spectrum. Relating specific colors to a region of wavelengths enables the differentiation between different tones, hues, and shades. It is possible for many different spectral distributions to produce identical color sensations (a phenomenon known as metamers). For example, a yellow color sensation may be caused by a single wavelength of light, for instance 590 nanometers, or it may be the result of viewing two equal amounts of light having individual wavelengths, such as 580 and 600 nanometers. It is also possible to view the color yellow as a narrow distribution encompassing all wavelengths between 580 and 600 nanometers. With regards to the human visual system, the same argument holds for all colors in the visible spectrum. However, recent studies indicate that some species (most notably, birds) can discriminate between colors perceived as metamers by humans.
Incandescent Light Sources
Early humans were without a reliable source of light during the long nights, but they could occasionally find and collect burning wood from bush fires, and then keep the flames blazing in a campfire for a short period of time. As knowledge progressed, man discovered that sparks, and subsequently fire, could be generated by striking certain stones together (such as flint and iron pyrite) or by aggressively rubbing wood against wood. Once these techniques were mastered, man could produce fire whenever it was desired.
When a fire burns, chemical energy is released in the form of heat and light. The burning fuel, whether it is grass, wood, oil, or some other combustible material, emits gases that are heated by the enormous chemical energy generated during combustion, making atoms in the gas glow or incandesce. Electrons within the gas atoms are promoted to higher energy levels by the heat, and light is released in the form of photons when the electrons relax to their ground state. The color of a flame is an indication of the temperature and how much energy is being released. A dull yellow flame is much cooler than a bright blue flame, but even the coolest flame is still very hot (at least 350 degrees Celsius).

Although tar and rags were employed to produce early torches, the first practical step in controlling fire occurred when the oil lamp was invented. Early lamps over 15,000 years old (Figure 2) have been discovered, made from rocks and shells, which burned animal fat and plant oils. Before gas lighting was invented, there was a tremendous demand for animal oil. The primary source of this oil was the tallow produced by boiling down fat tissues obtained from sea animals, such as whales and seals. Oil lamps eventually evolved into candles that were formed by casting hardened tallow or beeswax, as illustrated in Figure 2. Early candles generated quite a bit of smoke, but not much light. Eventually, it was discovered that paraffin wax, when properly cast with an impregnated cloth wick, produced a relatively bright flame without a significant amount of smoke.
During the nineteenth century, natural gas lighting became widespread throughout many of the major towns and cities of Europe, Asia, and the United States. Early gaslights operated by producing a jet of burning gas (a quite dangerous situation), while later models were fitted with a mantle, or fine net of chemically treated fabric, which disperses the flame and emits a much brighter light.
| Interactive Tutorial | |||||||||||
|
|||||||||||
Early microscopists relied on candles, oil lamps, and natural sunlight to provide illumination for the relatively crude optical systems in their microscopes. These primitive light sources suffered from flickering, uneven illumination, glare, and often were a potential fire hazard. Today, incandescent high-intensity tungsten-based lamps are the primary light source utilized in modern microscopes and the majority of household lighting systems.
Presented in Figure 3 are spectral distribution curves demonstrating the relative amounts of energy versus wavelength for several different sources of white light (comprised of a mixture containing all or most of the colors in the visible spectrum). The red curve represents the relative energy of tungsten light over the entire visible spectrum. As is apparent from examining the figure, the energy of tungsten light increases as wavelength increases. This effect dramatically influences the average color temperature of the resultant light, especially when it is compared to that of natural sunlight and fluorescent light (the mercury vapor lamp). The spectrum represented by a yellow curve profiles the visible light distribution from the natural sunlight spectrum sampled at noon. Under normal circumstances, sunlight contains the greatest amount of energy, but the curves illustrated in Figure 3 have all been normalized to the tungsten spectrum in order to ease comparison. The dark blue spectral curve is characteristic of a mercury arc lamp, and exhibits some notable differences from the tungsten and natural sunlight spectra. Several energy peaks are present in the discharge arc lamp spectrum that occur a result of superposed individual line spectra originating from the mercury vapor.
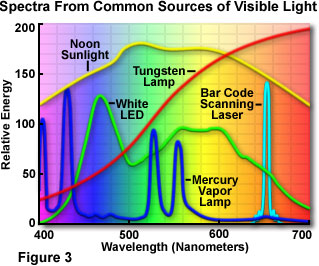
The visible light spectrum produced by a white light emitting diode (LED) is represented by the green curve in Figure 3. Light emitting diodes are inherently monochromatic devices, with the color being determined by the band gap between various semiconductor materials utilized in diode construction. Red, green, yellow, and blue diodes are common, and extensively employed as indicator lights for computers and other consumer electronics devices, such as radio tuners, television receivers, compact disk players, videocassette recorders, and digital videodisk players. White light LEDs are fabricated from gallium nitride blue diodes by coating the semiconductor die with a phosphor material, which emits a broad range of visible wavelengths when excited by light emitted from the blue diode. Laser spectra, whether derived from diodes or gas lasers, are characteristically very narrow, often comprising only one or a few specific wavelengths. An example is illustrated in Figure 3 (the cyan curve) for a low-current semiconductor diode laser that is useful for a variety of applications, including reading barcodes and tracking optical disk data.
Tungsten light sources are commonly termed incandescent, because they radiate light when heated by electrical energy. The filaments of modern light bulbs (or lamps) are generally composed of tungsten, a metal that is somewhat efficient at radiating light when resistively heated by an electrical current. Modern incandescent lamps descended from the carbon arc lamps invented by Sir Humphrey Davy, which produce light by a discharge arc formed between two carbon rods (or filament electrodes) when an electric potential is placed across the electrodes. Ultimately, the carbon arc lamp gave way to the first lamps that utilized carbon filaments contained in an evacuated glass envelope. Tungsten filaments, pioneered in 1910 by William David Coolidge, evaporate much more slowly than cotton-derived carbon fibers when heated in the vacuum of a glass envelope. The filament acts as a simple resistor, and emits a significant amount of light in addition to the heat generated by current flow.
| Interactive Tutorial | |||||||||||
|
|||||||||||
Tungsten incandescent lamps are thermal radiators that emit a continuous spectrum of light extending from about 300 nanometers, in the ultraviolet region, to about 1400 nanometers, in the near infrared region. Their design, construction, and operation are very simple, and a wide variety of these lamps have been utilized as incandescent light sources. Typical lamps consist of an sealed glass envelope (see Figure 4), evacuated or filled with an inert gas, and containing a tungsten wire filament that is energized by either direct or alternating current. The bulbs produce a tremendous amount of light and heat, but the light accounts for only 5 to 10 percent of their total energy output.
Tungsten lamps tend to suffer several drawbacks, such as a decreased intensity with age and a blackening of the inside envelope surface as evaporated tungsten is slowly deposited onto the glass. The color temperature and luminance of tungsten lamps vary with the applied voltage, but average values for color temperature range from about 2200 K to 3400 K. The surface temperature of an active tungsten filament is very high, typically averaging 2,550 degrees Celsius for a standard 100-watt commercial light bulb. In some cases, tungsten bulb envelopes are filled with the Noble gases krypton or xenon (inert fill gas) as an alternative to creating a vacuum in order to protect the hot tungsten filament. These gases improve the efficiency of incandescent lamps because they reduce the amount of evaporated tungsten that is deposited on the interior of the surrounding glass vessel.

Halogen bulbs, a high-performance version of the incandescent tungsten lamp, typically contain traces of iodine or bromine in the fill gas, which return evaporated tungsten to the filament far more efficiently than lamps made with other gases. Tungsten-halogen lamps, first developed by General Electric in the 1950s for lighting the tips of supersonic jet wings, are capable of producing very uniform bright light throughout the bulb lifetime. In addition, halogen lamps are much smaller and more efficient than tungsten lamps of comparable intensity. The lifetime of a tungsten-halogen bulb can be as much as 10 years under the most ideal conditions.
The filaments of tungsten-halogen lamps are often very compact spiral assemblies mounted in a borosilicate-halide glass (often termed fused quartz) envelope. High operating temperatures restrict the use of tungsten-halogen bulbs to well-ventilated lamphouses with fan-shaped heat sinks to eliminate the tremendous amount of heat generated by these bulbs. Many household lamps are equipped to operate with 300-500 watt tungsten-halogen lamps, and produce a significant amount light that fills a room much better than their weaker-emitting tungsten counterparts. When coupled with fiber optic light pipes and absorption or dichromatic filters, tungsten-halogen lamphouses provide high intensity illumination for a wide variety of optical microscopy applications, but as a major disadvantage, produce significant amounts of infrared light in the form of radiant heat that can easily degrade the specimen.
Fluorescent Light Sources
There are a wide variety of non-incandescent visible light sources that are employed for indoor and outdoor lighting, in addition to having important applications in optical microscopy. Most of these light sources are based on electric discharge through a gas such as mercury, or the Noble gases neon, argon, and xenon. The generation of visible light in gas discharge lamps relies on collisions between atoms and ions in the gas with an electrical current that is passed between a pair of electrodes placed at the ends of the bulb envelope.
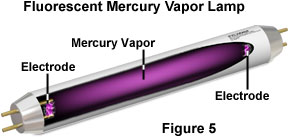
The glass tube of a common fluorescent lamp is coated with phosphor on the inside surface of the glass, and the tube is filled with mercury vapor at very low pressure (see Figure 5). An electric current is applied between the electrodes at the ends of the tube, producing a stream of electrons that flow from one electrode to the other. When electrons from the stream collide with mercury atoms, they excite electrons within the atoms to a higher energy state. This energy is released in the form of ultraviolet radiation when electrons in the mercury atoms return to the ground state. The ultraviolet radiation subsequently energizes the internal phosphor coating, causing it to emit the bright white light that we observe from fluorescent lights. Fluorescent lamps are about two to four times more efficient at emitting visible light, produce less waste heat, and typically last ten to twenty times longer than incandescent lamps.
A unique feature of fluorescent light sources is that they generate a series of wavelengths that are often concentrated into narrow bands termed line spectra. As a consequence, these sources do not produce the continuous spectrum of illumination that is characteristic of incandescent sources. A good example of a (almost exclusively) single wavelength source of non-incandescent visible light is the sodium-vapor lamps commonly employed in street lighting. These lamps emit a very intense yellow light, with over 95 percent of the emission being composed of 589-nanometer light and virtually no other wavelengths present in the output. It is possible to design gas-discharge lamps that will emit a nearly continuous spectrum in addition to the line spectra inherent in most of these lamps. The most common technique is to coat the inside surface of the tube with phosphor particles, which will absorb radiation emitted by the glowing gas and convert it into a broad spectrum of visible light ranging from blue to red.
Under normal circumstances, most individuals are not able to discern the difference between a line spectrum and a spectrum of continuous wavelengths. However, some objects reflect unusual colors in light from a discontinuous source, particularly under fluorescent lighting. This is why clothing, or other highly colored items, purchased in a store illuminated by fluorescent light often appears a slightly different color under natural sunlight or continuous tungsten illumination.
| Interactive Tutorial |
|
||||||||||
|
|||||||||||
In reflected light stereomicroscopy, particularly when examining heat-sensitive specimens, fluorescent lamps are favored over tungsten lamps because of their high efficiency and low heat output. Modern fluorescent lamps can be configured for linear tube or ring illuminators to provide the microscopist with intense, diffuse light. This source of artificial white light rivals sunlight (without the accompanying heat) in color temperature, and eliminates the flicker characteristics typical of consumer-grade fluorescent tubes. In comparison to tungsten, tungsten-halogen, or arc lamps, fluorescent-lamp microscope illuminators can provide relatively long periods (approximately 7,000 hours) of high quality service. As a diffuse light source, fluorescent lamps produce an evenly illuminated field of view without annoying hot spots or glare. Newer cold cathode illumination technology shows promise as a specialized light source in optical microscopy, particularly for short-lived events enhanced by fluorescence excitation, and for applications where waste heat or warm-up time in a light source may interfere with the specimen or the event being observed.
A specialized method for photographing moving specimens, particularly useful in darkfield microscopy illumination, has been devised using electronic photography flash systems. Electronic flash units operate through ionization in a xenon gas-filled glass envelope driven by the discharge of a large capacitor. The short-lived, high-voltage pulse from a transformer induces the xenon gas to ionize, allowing the capacitor to discharge through the now-conductive gas. A sudden burst of bright light is emitted, after which the xenon gas rapidly returns to a non-conductive state, and the capacitor recharges. Flash tubes provide 5,500 K illumination in an instantaneous burst that can capture a significant amount of object detail for spectacular results in photography, digital imaging, and photomicrography.
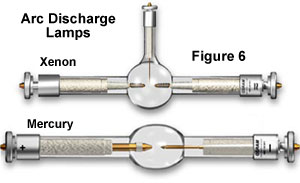
Arc discharge lamps, filled with gases such as mercury vapor and xenon, are favored sources of illumination for some specialized forms of fluorescence microscopy. A typical arc lamp is 10-100 times brighter than tungsten-based counterparts and can provide brilliant monochromatic illumination when combined with specially coated dichromatic interference filters. Unlike tungsten and tungsten-halogen lamps, arc lamps do not contain a filament, but rather, depend on ionization of the gaseous vapor though a high-energy arc discharge between two electrodes to produce their intense light. In general, arc lamps have an average lifetime of about 100-200 hours, and most external power supplies are equipped with a timer that enables the microscopist to monitor how much time has elapsed. Mercury arc lamps (often referred to as burners; see the mercury and xenon lamps illustrated in Figure 6) range in power from 50 to 200 watts and usually consist of two electrodes sealed under high mercury vapor pressure in a quartz glass envelope.
Mercury and xenon arc lamps do not provide even illumination intensity across the entire wavelength spectrum from near ultraviolet to infrared. Much of the intensity of the mercury arc lamp is expended in the near-ultraviolet and blue spectrum, with most of the high-intensity peaks occurring in the 300-450 nanometer range, except for a few higher-wavelength peaks in the green spectral region. In contrast, xenon arc lamps have a broader and more even intensity output across the visible spectrum, and do not exhibit the very high-spectral-intensity peaks that are characteristic of mercury lamps. Xenon lamps are deficient in the ultraviolet, however, and expend a large proportion of their intensity in the infrared, requiring care in control and elimination of excess heat when these lamps are employed.
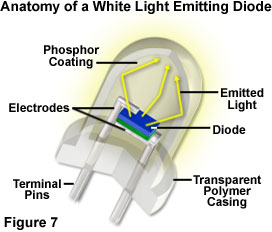
The era of utilizing light emitting diodes as a practical source of illumination has arrived with the twenty-first century, and the diode is an ideal complement to the union of semiconductor technology and optical microscopy. The relatively low power consumption (1 to 3 volts at 10 to 100 milliamperes), and long working life of light emitting diodes, renders these devices perfect light sources when low to medium intensity levels of white light are required. Microscopes connected to computers interfaced through a universal serial bus (USB) port, or powered by batteries, can utilize the LED as a small, low-heat, low-power, and low-cost internal light source for visual observation and digital image capture. Several teaching and entry-level research microscopes currently utilize an internal, high-intensity white light emitting diode that serves as the primary light source.
Although the epoxy envelope light projection characteristics are still being explored, light emitting diodes are currently being tested and marketed in a wide variety of applications, such as traffic signals, signs, flashlights, and external ring-style illuminators for microscopy. The light produced by white LEDs has a color temperature spectrum similar to that of a mercury vapor lamp, which is in the daylight illumination category. Examining the white LED emission spectrum presented in Figure 3, the transmission peak at 460 nanometers is due to blue light emitted by the gallium nitride diode semiconductor, while the broad high-transmission range positioned between 550 and 650 nanometers results from secondary light emitted by a phosphor coating inside the polymer jacket. The combination of wavelengths produces "white" light having a relatively high color temperature, which is a suitable wavelength range for imaging and observation in optical microscopy.
Laser Light Sources
Another source of visible light that is becoming increasingly more important in our everyday lives is laser illumination. The acronym LASER is an abbreviation for Light Amplification by the Stimulated Emission of Radiation. Among the unique features of lasers is that they emit a continuous beam of light composed of a single discrete wavelength (or sometimes several wavelengths) that exits the device in a single, aligned phase, commonly termed coherent light. The wavelength of light emitted by a laser depends upon the material from which the laser crystal, diode, or gas is composed. Lasers are produced in a variety of shapes and sizes, ranging from tiny diode lasers small enough to fit through the eye of a needle, to huge military and research-grade instruments that fill an entire building.

Lasers are used as light sources in a number of applications ranging from compact disk readers to measuring tools and surgical instruments. The familiar red light of the helium-neon (often abbreviated He-Ne) laser scans consumer purchases by lighting optical bar codes, but also plays a critical role in many laser scanning confocal microscopy systems. The application of lasers in optical microscopy is also growing in importance, both as a sole light source, and in combination with fluorescent and/or incandescent light sources. Despite the relatively high cost, lasers find particularly wide application in fluorescence, monochromatic brightfield, and in the rapidly growing fields of laser scanning confocal, total internal reflection, fluorescence resonance energy transfer, and multi-photon microscopy.
| Interactive Tutorial |
|
||||||||||
|
|||||||||||
Argon-ion lasers (Figure 8) produce powerful spectral emissions at 488 and 514 nanometers, while krypton gas lasers exhibit large peaks at wavelengths of 647.1 and 752.5 nanometers. Both of these lasers are often utilized as excitation sources in laser scanning confocal microscopy. Titanium-doped sapphire crystal mode-locked pulsed lasers are used as sources for multiphoton excitation due to their high peak intensity, but they also feature low average power and short duty cycles. As preferred light sources for multiphoton microscopy, pulsed lasers are considerably more expensive and difficult to operate than the small, air-cooled lasers employed in confocal microscopy.
Newer laser technology features semiconductor-based laser diodes and single on-chip lasers that reduce the size and power requirements for light sources. Laser diodes, such as neodymium:yttrium lithium fluoride (Nd:YLF) and neodymium:yttrium vanadate (Nd:YVO(4)), typically are much faster in response than LEDs, but are also relatively small and require little power. Disadvantages of using lasers in microscopy include additional costs for the light source, the risk of expensive damage to optics, increased costs associated with lens and mirror coatings, destruction of specimens, and potential retinal damage to the microscopist if safe handling and operating techniques are ignored.
From this discussion, it is apparent that although there are a wide variety of available illumination sources, we generally rely on only a few throughout our everyday lives. During daylight hours the sun serves as our main source of illumination outdoors, while we generally rely on fluorescent and tungsten lighting while indoors and during the evening hours. As discussed above, these three primary lighting sources all have different properties and spectral characteristics, but their maximum intensities all fall within the visible light range. The human brain adjusts automatically to the different light sources, and we interpret the colors of most objects around us as hardly changing when they are viewed under differing conditions of illumination.
Contributing Authors
Kenneth R. Spring - Scientific Consultant, Lusby, Maryland, 20657.
Michael W. Davidson - National High Magnetic Field Laboratory, 1800 East Paul Dirac Dr., The Florida State University, Tallahassee, Florida, 32310.
BACK TO SOURCES OF VISIBLE LIGHT
