Interactive Tutorials
Photosynthesis
Green plants absorb water and carbon dioxide from the environment, and utilizing energy from the sun, turn these simple substances into glucose and oxygen. With glucose as a basic building block, plants synthesize a number of complex carbon-based biochemicals used to grow and sustain life. This process is termed photosynthesis, and is the cornerstone of life on Earth. The tutorial demonstrates the basic molecular steps in the photosynthetic process.
The tutorial initializes with water molecules being converted to molecular hydrogen and oxygen as a result of photon absorption in the granum. Subsequently, the hydrogen molecules react with carbon dioxide in the stroma to produce oxygen and carbohydrates. A legend for the individual molecule graphics is presented in the lower portion of the window. The tutorial operates without user intervention, but the speed can be adjusted with the Applet Speed slider.
English chemist Joseph Priestley was the first investigator to find that plants release oxygen when they are healthy and growing. His experiments documented the process of photosynthesis, and showed that respiration and photosynthesis are related processes, but work in opposite directions. Priestley's most famous experiment (around 1772) demonstrated that a candle would quickly extinguish if placed in a bell jar, but would burn again in the same air if a plant were left in the container for several days. He concluded that the plant had "restored" air that had been "injured" by burning the candle. In further experiments Priestley demonstrated that a mouse placed in the jar would "injure" the air in the same way as a candle, but could then breathe the air after it was "restored", leading to the concept that respiration and photosynthesis are opposite processes. In Priestley's words, "the air would neither extinguish a candle, nor was it at all inconvenient to a mouse which I put into it". Priestley had discovered a substance that would later be named oxygen by the French chemist Antoine Laurent Lavoisier, who extensively investigated the relationship between combustion and air.
A key component to the understanding of photosynthesis was still missing until Dutch physiologist Jan Ingenhousz determined in 1778 that plants only absorb carbon dioxide and release oxygen when they are exposed to light. Finally, German physicist, Julius Robert Mayer, formalized the concept that energy was being transformed from light to produce new chemicals in growing plants. Mayer believed that a specialized chemical process (now known as oxidation) was the ultimate source of energy for a living organism.
Photosynthesis, meaning "putting together by light", is the process by which almost all plants, some bacteria, and a few protistans harness the energy in sunlight to produce sugar (and oxygen as a byproduct). The conversion of light energy into chemical energy is dependent on the substance chlorophyll, a green pigment that bestows upon plant leaves their green appearance. Not all plants have leaves, but the ones that do are very efficient at converting solar into chemical energy. As such, leaves can be thought of as biological solar collectors, equipped with numerous tiny cells that carry out photosynthesis on a microscopic level.
A pigment is defined as any substance that absorbs and reflects visible light. A majority of pigments are coloring agents, displaying specific colors that are dependent upon the wavelength distribution of light reflected and absorbed. Each pigment has its own characteristic absorption spectrum, which determines the portion of the spectrum over which the pigment is effective at collecting energy from light. Chlorophyll, a biochemical that is common to all photosynthetic organisms, reflects green (intermediate) wavelengths, and absorbs energy from the violet-blue and reddish-orange wavelengths at opposite ends of the visible light spectrum.
Chlorophyll is a complex molecule that exists in several modifications or isomers in plants and other photosynthetic organisms. All organisms that carry out photosynthesis contain the variety known as chlorophyll a. Many other organisms also contain accessory pigments, including other chlorophylls, carotenoids, and xanthophylls, which absorb other wavelengths in the visible spectrum. In this manner, plants may be tailored for specific environmental factors that affect the nature of the light available to them in their particular niche. Factors such as water depth and quality strongly influence the wavelengths of light available in different aquatic and marine environments, and play a large role in the photosynthetic function of different phytoplankton and other protistan species.
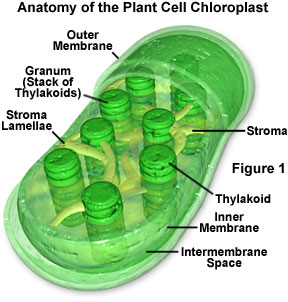
When a pigment absorbs light energy, the energy can either be dissipated as heat, emitted at a longer wavelength as fluorescence, or it can trigger a chemical reaction. Certain membranes and structures in photosynthetic organisms serve as the structural units of photosynthesis because chlorophyll will only participate in chemical reactions when the molecule is associated with proteins embedded in a membrane (such as chloroplasts, for example; Figure 1). Photosynthesis is a two-stage process, and in organisms that have chloroplasts, two different areas of these structures house the individual processes. A light-dependent process (often termed light reactions) takes place in the grana, while a second light-independent process (dark reactions) subsequently occurs in the stroma of chloroplasts (see Figure 1). It is believed that the dark reactions can take place in the absence of light as long as the energy carriers developed in the light reactions are present.
The first stage of photosynthesis occurs when the energy from light is directly utilized to produce energy carrier molecules, such as adenosine triphosphate (ATP). In this stage, water is split into its components, and oxygen is released as a by-product. The energized transportation vehicles are subsequently utilized in the second and most fundamental stage of the photosynthetic process: production of carbon-to-carbon covalent bonds. The second stage does not require illumination (a dark process), and is responsible for providing the basic nutrition for the plant cell, as well as building materials for cell walls and other components. In the process, carbon dioxide is fixed along with hydrogen to form carbohydrates, a family of biochemicals that contain equal numbers of carbon atoms and water molecules. Overall, the photosynthetic process does not allow living organisms to directly utilize light energy, but instead involves energy capture in the first stage followed by a second stage of complex biochemical reactions that converts the energy into chemical bonds.
Contributing Authors
Matthew J. Parry-Hill, Robert T. Sutter and Michael W. Davidson - National High Magnetic Field Laboratory, 1800 East Paul Dirac Dr., The Florida State University, Tallahassee, Florida, 32310.
