Interactive Tutorials
Hydrogen Fuel Cell Basics
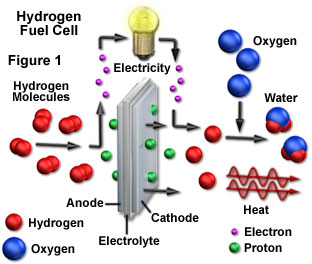
Fuel cells are designed to utilize a catalyst, such as platinum, to convert a mixture of hydrogen and oxygen into water. An important byproduct of this chemical reaction is the electricity generated when hydrogen molecules interact (through oxidation) with the anode to produce protons and electrons. This interactive tutorial explores the major steps in fuel cell operation.
The tutorial initializes with a hydrogen fuel cell having hydrogen and oxygen input chambers separated by a cathode, anode, and an electrolyte. When the hydrogen molecules (represented by red spheres) encounter the anode, they are ionized to produce positively charged protons and two electrons (green spheres) per molecule. Note that although two separate red hydrogen spheres represent a single molecule of hydrogen in the tutorial, in reality, a molecule of hydrogen contains two individual hydrogen atoms that share a covalent bond. The electrons generated by the oxidation of hydrogen are utilized to provide current for a light bulb, and flow into the cathode where they reduce a combination of oxygen (blue spheres) and hydrogen into water, thus completing the reaction. The Applet Speed slider can be utilized to increase or decrease the speed of events in the tutorial.
Although solar energy exists in an inexhaustible supply that is available at no cost (and is non-polluting), the conversion of light energy from the sun is associated with numerous problems that severely limit efficient applications. The most desirable situation would be to devise a mechanism to convert solar energy into a compact and portable form that could easily be transported to distant locations. Many research endeavors are aimed at using concentrated solar energy to achieve the high temperatures necessary to drive various chemical reactions, often using chemical catalysts to produce different combinations of gaseous fuels that can be easily stored and transported. Some of the possibilities are promising, but most experts in the field of energy conversion agree that the ultimate fuel to be derived from solar energy conversion is hydrogen.
The appeal of hydrogen as a fuel is overwhelming. Molecular hydrogen is made from the lightest element in the universe, and can easily be stored and transported. Furthermore, hydrogen can be derived from water with molecular oxygen as the only byproduct. When hydrogen is burned, it combines with oxygen in the air to form water once again, thereby regenerating the source material. Most importantly, during the entire cycle that releases energy in a usable form, none of the intermediate steps produces significant quantities of pollutants. As long as the sun continues to produce its light energy, the hydrogen supply is inexhaustible. Currently, hydrogen is used primarily as a rocket fuel (in the form of catalytic fuel cells as illustrated in Figure 1), and as a component of a number of industrial chemical processes. However, with readily achieved modifications, the smallest element could fulfill all of mankind's electricity and transportation requirements.
Although hydrogen can be produced directly from water, some form of energy input is required to carry out the separation from oxygen. One means of driving the reaction is to use an electric current in a process termed electrolysis, and sunlight can be used to generate the electricity for the process. Electrolysis involves an oxidation-reduction reaction in which current passed through an electrode pair immersed in water produces hydrogen and oxygen gases at the opposite electrodes. Another possible route for hydrogen generation is to concentrate sunlight at high enough temperatures to cause the thermal decomposition of water into its oxygen and hydrogen components, which can then be separated.
Ultimately, a more sophisticated means of splitting water to make use of the hydrogen would be desirable. One technique from which the separation might be achieved is to harness the sun's energy through chemical reactions in a manner similar to the photosynthesis process used by plants and bacteria. When they are exposed to sunlight, green chlorophyll-containing plants continually split water molecules, releasing oxygen and combining hydrogen with carbon dioxide to form sugars. If the first part of this, or a similar process, can be duplicated, a limitless supply of hydrogen would be available, driven by solar energy input.
A significant effort is focused on the development of artificial photosynthesis, which on a fundamental level, can be described as photo-induced charge separation at molecularly defined interfaces. One of the desired goals of this research is the development of light-controlled enzymes and even molecular-scale electronics, which involve the transfer of charge carriers in response to light and chemical activity. Another objective of such research is the biotechnological production of substances such as enzymes and pigments. In recent years bacteria and similar organisms that degrade oil have been utilized to clean up spills. Currently, scientists are attempting to perfect ways of using organisms that live and grow on solar energy for a variety of bioremediation purposes, such as cleaning up contaminated water supplies.
Under certain conditions, algae can be induced to switch off their normal photosynthetic sequence at a particular stage and produce significant amounts of hydrogen. By preventing the cells from burning stored fuel in the usual manner, the algae are forced to activate an alternative metabolic pathway that results in production of hydrogen in significant amounts. This discovery raises hopes that someday hydrogen fuel can be produced from sunlight and water through the photosynthetic process using large-scale photobioreactor complexes. Recent investigations have uncovered marine bacteria that contain the light-absorbing pigment proteorhodopsin, which enables them to convert sunlight into cellular energy without relying on chlorophyll. This discovery raises the possibility of using easily manipulated bacteria, such as E. coli, in light-driven energy generators having numerous applications in both the physical and life sciences.
Contributing Authors
Matthew J. Parry-Hill, Robert T. Sutter and Michael W. Davidson - National High Magnetic Field Laboratory, 1800 East Paul Dirac Dr., The Florida State University, Tallahassee, Florida, 32310.
