Polarized Light Digital Image Gallery
Vitamin E (alpha-Tocopheryl)
Known to millions as vitamin E, d-alpha-tocopheryl acetate is a fat-soluble vitamin, which is stored in the liver, muscle, adipose tissue, red blood cells, and several vital organs and glands. Vitamin E, a strong antioxidant, plays a starring role in protecting body tissues from damaging free radicals as well as critical functions in cellular respiration and for prolonging the life of red blood cells.
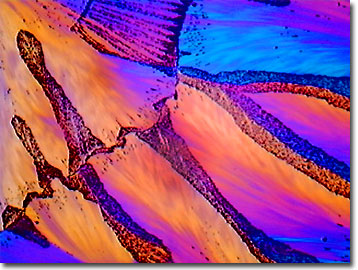
View a second image of crystallized Vitamin E.
Naturally, vitamin E occurs in wheat germ oil, nuts, seeds, vegetable oils, whole grains, egg yolks, and leafy green vegetables. Discovered in 1922 by American anatomists and physiologists Herbert McLean Evans and Katherine Scott Bishop, alpha-tocopheryl was known as food factor X and found essential for rat pregnancy. In the same year, food factor X was discovered in yeast and lettuce. By 1924, it was renamed vitamin E and in 1936, Evans and his colleagues extracted and isolated alpha-tocopheryl from wheat germ oil. Synthesis was completed in 1938 by the Swiss Nobel laureate for Chemistry, Paul Karrer, and in 1968, the United States Food and Drug Administration set the recommended dietary (or daily) allowance (RDA) for vitamin E at a conservative 20 milligrams or 30 international units (IU). Over-the-counter synthesized forms as dl-alpha-tocopheryl acetate (or succinate) are often 200 international units (or 165 milligrams) or 400 international units (13.3 times the recommended daily allowance), with a dosage of one capsule per day.
Research on the many uses of vitamin E started in 1950 with its use in a topical skin cream formulation for treating frostbite, and then as antioxidant fighting free radicals and reducing the risks of heart disease. Vitamin E is unstable when exposed to heat, light, and oxygen, but may help prevent and fight cancers, protect cell membranes from breaking down, and may play a role in therapy for Alzheimer's patients. Interestingly, alpha-tocopheryl helps the body effectively use and store vitamin A and protects B-complex and vitamin C from oxidation reactions. The human body does not make its own vitamin E, so it must be taken from nutritional sources or as a dietary supplement. While the acetate form is fat soluble, the succinate compound is water-soluble. As a crystalline solid, d-alpha-tocopheryl acetate has a melting point of 28 degrees Celsius and a boiling point of 184 degrees Celsius. Alpha-tocopheryl is only one of a group of the lipid-soluble compounds known as tocopherols and tocotrienols (or tocols), but it is considered the most biopotent or powerful. The synthesized version, dl-alpha-tocopheryl acetate exists in equal amounts of eight isomers while the natural extraction from vegetable oils, d-alpha-tocopheryl acetate, exists only as one isomer. Some indications are that the natural version is a better alternative to the synthesized form of the vitamin for increasing levels in body tissues and extending retention time.
Contributing Authors
Omar Alvarado, Thomas J. Fellers and Michael W. Davidson - National High Magnetic Field Laboratory, 1800 East Paul Dirac Dr., The Florida State University, Tallahassee, Florida, 32310.
BACK TO THE POLARIZED LIGHT IMAGE GALLERY
BACK TO THE DIGITAL IMAGE GALLERIES
Questions or comments? Send us an email.
© 1995-2025 by Michael W. Davidson and The Florida State University. All Rights Reserved. No images, graphics, software, scripts, or applets may be reproduced or used in any manner without permission from the copyright holders. Use of this website means you agree to all of the Legal Terms and Conditions set forth by the owners.
This website is maintained by our
Graphics & Web Programming Team
in collaboration with Optical Microscopy at the
National High Magnetic Field Laboratory.
Last Modification Friday, Nov 13, 2015 at 01:19 PM
Access Count Since September 17, 2002: 8819
Visit the website of our partner in introductory microscopy education:
|
|
