The Frequency and Wavelength of Light
Although visible light acts as a wave in some respects, it also displays properties characteristic of particles. The particle-like properties of visible light are exhibited through small, energy-bearing entities known as photons.
The source of visible light and all other forms of electromagnetic radiation is the atom. This is due to fact that electrons moving in orbits around the nucleus of an atom are arranged in different energy levels within their electron clouds. These electrons can absorb additional energy from outside sources of electromagnetic radiation, which results in their promotion to a higher energy level or electron cloud. As illustrated in Figure 1, this promotion is an inherently unstable situation. The electron eventually loses the extra energy by emitting photons and, in doing so, the electron falls back into its original and stable energy level.
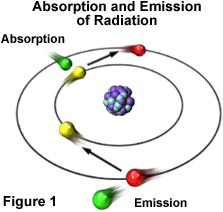
The energy of emitted photons equals the energy that was originally absorbed by the electron minus any other lost energy. Thus, the energy of photons can vary to a great degree since it is dependent upon the energy of source electrons. For instance, the electrons may come from radio waves, which contain much less energy than do microwaves, infrared rays, or visible light, or from a source that contains a much greater amount of energy, such as ultraviolet light or X-rays. As a rule, higher energies are associated with shorter wavelengths and lower energies are associated with higher wavelengths.
| Interactive Java Tutorial | |||||||||||
|
|||||||||||
Figure 2 is designed to clarify some of the terminology used in reference to electromagnetic radiation. The image illustrates an electromagnetic wave that propagates, or travels, in a direction from upper left to lower right. This wave travels at the speed of light and is known as a transverse wave, in which the direction of wave energy lies at right angles to the direction of propagation.
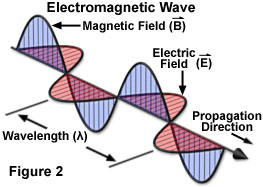
The wave in Figure 2 is generating both electric and magnetic oscillating fields that are oriented at 90-degree angles with respect to each other and also to the direction of energy. The distance between two successive peaks of the wave is equivalent to the wavelength of the radiation. The frequency of the radiation, however, is determined by the number of oscillations per second, which is usually measured in hertz, or cycles per second.
| Interactive Java Tutorial | |||||||||||
|
|||||||||||
An increase in frequency produces a proportional decrease in the wavelength of light, with a corresponding increase in the energy of the photons that comprise the light. Thus, the relationship between the wavelength of light and its frequency in inversely proportional and can be illustrated by a simple equation:
where c is the speed of light with a constant value of 300 million meters per second, n is the frequency of the light in hertz (Hz) or cycles per second, and l is the wavelength of the light in meters. Upon entering a new medium, such as glass or water, the speed and wavelength of light is reduced, although the frequency remains unaltered.
The energy of a photon, however, is directly proportional to its frequency and inversely proportional to its wavelength. This relationship can be symbolized by another simple equation:
where E is the energy in kiloJoules per mole, h is Planck's constant with a value of 6.626 x E-34 Joule-seconds per particle, and the other variables are defined as above. Thus, as frequency increases, the energy of emitted photons increases. The reverse is also true. As the frequency of radiation decreases, there is a corresponding decrease in the energy of emitted photons.
| Interactive Java Tutorial | |||||||||||
|
|||||||||||
Since the energy of photons depends upon the energy of source electrons, very high-frequency electromagnetic radiation, such as gamma rays, X-rays, and ultraviolet light, possess very short wavelengths and, therefore, a great deal of energy. On the other hand, lower frequency radiation, such as visible, infrared, microwave, and radio waves, have greater wavelengths, but correspondingly lower frequencies and energy. However, it is important to realize that though the electromagnetic spectrum of energy is commonly thought of as comprising about 24 orders of magnitude in frequency and wavelength, no intrinsic upper or lower boundaries exist.
Contributing Authors
Mortimer Abramowitz - Olympus America, Inc., Two Corporate Center Drive., Melville, New York, 11747.
Shannon H. Neaves and Michael W. Davidson - National High Magnetic Field Laboratory, 1800 East Paul Dirac Dr., The Florida State University, Tallahassee, Florida, 32310.
