The Nature of Electromagnetic Radiation
The light that can be perceived by the naked eye is only a small part of a larger family of wave-like energy known as electromagnetic radiation. The term is derived from the characteristic electric and magnetic properties common to all forms of electromagnetic radiation, which includes everything from high frequency gamma rays through X-rays, ultraviolet light, infrared radiation and microwaves to very low frequency radio waves. The standard unit of electromagnetic radiation measurement is the size of its wavelength (in vacuo), usually reported in terms of nanometers, which represent one-thousandth of a micrometer or 0.000000001 meter. The frequency of electromagnetic radiation, that is its number of oscillations per second, is proportional to the reciprocal of its wavelength. Thus, longer wavelengths correspond to lower frequency radiation and shorter wavelengths correspond to higher frequency radiation.
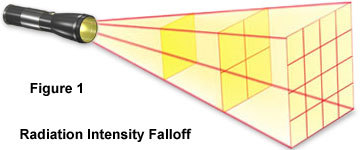
Though the wavelengths and frequencies of various forms of electromagnetic radiation differ, they are fundamentally similar in that they all travel in straight lines unless they are refracted or reflected, and at the same speed--approximately 186,000 miles per second, commonly known as the speed of light. Moreover, the intensity of electromagnetic radiation is inversely proportional to the square of the distance it travels, as illustrated above in Figure 1. In other words, after light has traveled 2x distance, the intensity decreases by a factor of four-fold.
| Interactive Java Tutorial | |||||||||||
|
|||||||||||
Gamma rays, which have the shortest wavelengths, and therefore the highest-frequency electromagnetic energy, are emitted by certain radioactive materials and also originate in outer space. These powerful rays have tremendous penetrating ability and have been reported to be able to pass through three meters of concrete. The wavelengths gamma rays exhibit range from 0.1 to 0.000001 nanometers.
X-rays have a frequency just above ultraviolet radiation and are powerful enough to pass easily through many materials, such as the soft tissues of animals. Consequently, X-rays are used extensively in the medical field to investigate textures in the human body. The frequency spectrum of X-rays covers a very large range with corresponding wavelengths extending from a nanometer to 0.00001 nanometers.
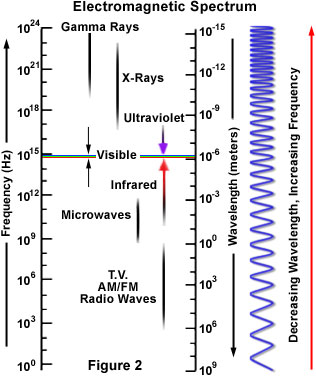
Ultraviolet radiation exhibits frequencies just above those of visible light, but at the high end of their frequency range, these rays have enough energy to kill living cells and cause tremendous tissue damage. For instance, the sun is a constant source of ultraviolet radiation and small doses of this light can promote the production of vitamin D and tan the skin. However, too much exposure to the ultraviolet radiation of the sun can lead to serious sunburn and skin cancer. Ultraviolet light is frequently utilized in scientific instruments and is also important in astronomical observations of the solar system, galaxy, and other parts of the universe. The ultraviolet wavelength spectrum ranges from approximately 50 to 350 or 400 nanometers.
Infrared radiation extends from the bottom portion of the visible spectrum, which is characterized by wavelengths of approximately 700 nanometers, to about one-millimeter wavelengths. This type of radiation is associated with the thermal region, where visible light is not necessarily present. For example, the human body does not emit visible light but it does emit infrared radiation, which is felt as heat. Almost all objects emit infrared rays, but the amount they emit is dependent on the temperature of the object. Warmer objects emit more infrared radiation than cooler objects. Common uses for infrared radiation are night vision scopes, electronic detectors, and sensors in satellites and airplanes.
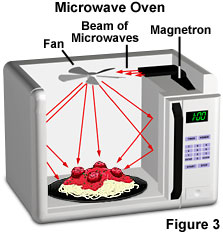
Microwave wavelengths range from approximately one millimeter to about thirty centimeters, or about one foot in length. The energy spectrum of microwaves is best known for its use in oven technology, in which their wavelengths are tuned to frequencies that are readily absorbed by water molecules. When the water in the food absorbs the energy, it releases heat and warms the food, as illustrated in Figure 3. The containers holding the food in microwave ovens usually remain cool, however, because they contain very little water. Microwaves are the highest frequency radio waves and are emitted by the Earth, buildings, cars, planes, and other large objects. Short wavelength microwaves are also the basis for RADAR (RAdio Detecting And Ranging), a technique used in locating large objects and calculating their speed and distance.
Radio waves range from wavelengths of less than a centimeter to tens and even hundreds of meters. Radio waves are well known for their ability to transmit radio and television signals, and when used for such purposes, generally consist of two types of transmissions: amplitude modulated (AM) waves that vary in the amplitude of the wavelengths and frequency modulated (FM) waves that vary in wavelength frequency. Frequency modulated radio waves are shorter in length than amplitude modulated waves and tend to be blocked by large objects such as houses, buildings, and tunnels. Amplitude modulated waves, however, are longer than frequency modulated waves and can be bent around these large objects to improve reception.
Within the visible light spectrum, which encompasses wavelengths of 400 to 700 nanometers, a rainbow of colors can be seen. The array of reds, blues, and greens may seem large at first, but, as demonstrated, they represent only a very small portion of the entire electromagnetic spectrum. Though the various other forms of electromagnetic radiation are invisible to the naked eye, they are constant, and important, factors in the milieu of the world.
Contributing Authors
Mortimer Abramowitz - Olympus America, Inc., Two Corporate Center Drive., Melville, New York, 11747.
Shannon H. Neaves and Michael W. Davidson - National High Magnetic Field Laboratory, 1800 East Paul Dirac Dr., The Florida State University, Tallahassee, Florida, 32310.
