Ribosomes
All living cells contain ribosomes, tiny organelles composed of approximately 60 percent ribosomal RNA (rRNA) and 40 percent protein. However, though they are generally described as organelles, it is important to note that ribosomes are not bound by a membrane and are much smaller than other organelles. Some cell types may hold a few million ribosomes, but several thousand is more typical. The organelles require the use of an electron microscope to be visually detected.
Ribosomes are mainly found bound to the endoplasmic reticulum and the nuclear envelope, as well as freely scattered throughout the cytoplasm, depending upon whether the cell is plant, animal, or bacteria. The organelles serve as the protein production machinery for the cell and are consequently most abundant in cells that are active in protein synthesis, such as pancreas and brain cells. Some of the proteins synthesized by ribosomes are for the cell's own internal use, especially those that are produced by free ribosomes. Many of the proteins produced by bound ribosomes, however, are transported outside of the cell.
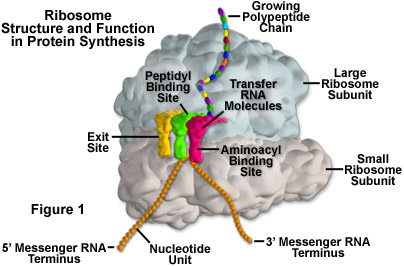
In eukaryotes, the rRNA in ribosomes is organized into four strands, and in prokaryotes, three strands. Eukaryote ribosomes are produced and assembled in the nucleolus. Ribosomal proteins enter the nucleolus and combine with the four rRNA strands to create the two ribosomal subunits (one small and one large) that will make up the completed ribosome (see Figure 1). The ribosome units leave the nucleus through the nuclear pores and unite once in the cytoplasm for the purpose of protein synthesis. When protein production is not being carried out, the two subunits of a ribosome are separated.
In 2000, the complete three-dimensional structure of the large and small subunits of a ribosome was established. Evidence based on this structure suggests, as had long been assumed, that it is the rRNA that provides the ribosome with its basic formation and functionality, not proteins. Apparently the proteins in a ribosome help fill in structural gaps and enhance protein synthesis, although the process can take place in their absence, albeit at a much slower rate.
The units of a ribosome are often described by their Svedberg (s) values, which are based upon their rate of sedimentation in a centrifuge. The ribosomes in a eukaryotic cell generally have a Svedberg value of 80S and are comprised of 40s and 60s subunits. Prokaryotic cells, on the other hand, contain 70S ribosomes, each of which consists of a 30s and a 50s subunit. As demonstrated by these values, Svedberg units are not additive, so the values of the two subunits of a ribosome do not add up to the Svedberg value of the entire organelle. This is because the rate of sedimentation of a molecule depends upon its size and shape, rather than simply its molecular weight.
Protein synthesis requires the assistance of two other kinds of RNA molecules in addition to rRNA. Messenger RNA (mRNA) provides the template of instructions from the cellular DNA for building a specific protein. Transfer RNA (tRNA) brings the protein building blocks, amino acids, to the ribosome. There are three adjacent tRNA binding sites on a ribosome: the aminoacyl binding site for a tRNA molecule attached to the next amino acid in the protein (as illustrated in Figure 1), the peptidyl binding site for the central tRNA molecule containing the growing peptide chain, and an exit binding site to discharge used tRNA molecules from the ribosome.
Once the protein backbone amino acids are polymerized, the ribosome releases the protein and it is transported to the cytoplasm in prokaryotes or to the Golgi apparatus in eukaryotes. There, the proteins are completed and released inside or outside the cell. Ribosomes are very efficient organelles. A single ribosome in a eukaryotic cell can add 2 amino acids to a protein chain every second. In prokaryotes, ribosomes can work even faster, adding about 20 amino acids to a polypeptide every second.
In addition to the most familiar cellular locations of ribosomes, the organelles can also be found inside mitochondria and the chloroplasts of plants. These ribosomes notably differ in size and makeup than other ribosomes found in eukaryotic cells, and are more akin to those present in bacteria and blue-green algae cells. The similarity of mitochondrial and chloroplast ribosomes to prokaryotic ribosomes is generally considered strong supportive evidence that mitochondria and chloroplasts evolved from ancestral prokaryotes.
