Microfilaments
Common to all eukaryotic cells, these filaments are primarily structural in function and are an important component of the cytoskeleton, along with microtubules and often the intermediate filaments. Microfilaments range from 5 to 9 nanometers in diameter and are designed to bear large amounts of tension. In association with myosin, microfilaments help to generate the forces used in cellular contraction and basic cell movements. The filaments also enable a dividing cell to pinch off into two cells and are involved in amoeboid movements of certain types of cells.
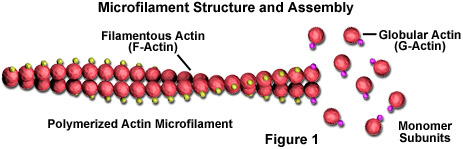
Microfilaments are solid rods made of a protein known as actin. When it is first produced by the cell, actin appears in a globular form (G-actin; see Figure 1). In microfilaments, however, which are also often referred to as actin filaments, long polymerized chains of the molecules are intertwined in a helix, creating a filamentous form of the protein (F-actin). All of the subunits that compose a microfilament are connected in such a way that they have the same orientation. Due to this fact, each microfilament exhibits polarity, the two ends of the filament being distinctly different. This polarity affects the growth rate of microfilaments, one end (termed the plus end) typically assembling and disassembling faster than the other (the minus end).
Unlike microtubules, which typically extend out from the centrosome of a cell, microfilaments are typically nucleated at the plasma membrane. Therefore, the periphery (edges) of a cell generally contains the highest concentration of microfilaments. A number of external factors and a group of special proteins influence microfilament characteristics, however, and enable them to make rapid changes if needed, even if the filaments must be completely disassembled in one region of the cell and reassembled somewhere else. When found directly beneath the plasma membrane, microfilaments are considered part of the cell cortex, which regulates the shape and movement of the cell's surface. Consequently, microfilaments play a key role in development of various cell surface projections (as illustrated in Figure 2), including filopodia, lamellipodia, and stereocilia.
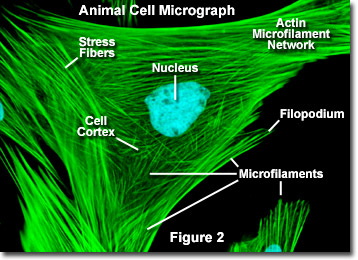
Illustrated in Figure 2 is a fluorescence digital image of an Indian Muntjac deer skin fibroblast cell stained with fluorescent probes targeting the nucleus (blue) and the actin cytoskeletal network (green). Individually, microfilaments are relatively flexible. In the cells of living organisms, however, the actin filaments are usually organized into larger, much stronger structures by various accessory proteins. The exact structural form that a group of microfilaments assumes depends on their primary function and the particular proteins that bind them together. For instance, in the core of surface protrusions called microspikes, microfilaments are organized into tight parallel bundles by the bundling protein fimbrin. Bundles of the filaments are less tightly packed together, however, when they are bound by alpha-actinin or are associated with fibroblast stress fibers (the parallel green fibers in Figure 2). Notably, the microfilament connections created by some cross-linking proteins result in a web-like network or gel form rather than filament bundles.
Over the course of evolutionary history of the cell, actin has remained relatively unchanged. This, along with the fact that all eukaryotic cells heavily depend upon the integrity of their actin filaments in order to be able to survive the many stresses they are faced with in their environment, makes actin an excellent target for organisms seeking to injure cells. Accordingly, many plants, which are unable to physically avoid predators that might want to eat them or harm them in some other way, produce toxins that affect cellular actin and microfilaments as a defensive mechanism. The death cap mushroom, for example, produces a substance called phalloidin that binds to and stabilizes actin filaments, which can be fatal to cells.
