Intermediate Filaments
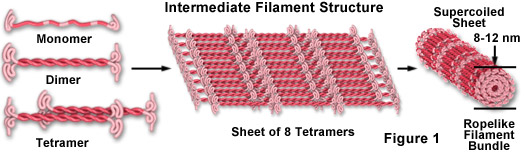
Intermediate filaments are a very broad class of fibrous proteins that play an important role as both structural and functional elements of the cytoskeleton. Ranging in size from 8 to 12 nanometers (in diameter; see Figure 1), intermediate filaments function as tension-bearing elements to help maintain cell shape and rigidity, and serve to anchor in place several organelles, including the nucleus and desmosomes. Intermediate filaments are also involved in formation of the nuclear lamina, a net-like meshwork array that lines the inner nuclear membrane and governs the shape of the nucleus.
Although all eukaryotes contain the common cytoskeletal elements actin and tubulin (both free in the cytoplasm and polymerized in the form of microfilaments and microtubules), intermediate filaments are found only in some metazoan species, including vertebrates, nematodes, and molluscs. In vertebrates, intermediate filament presence and composition is not only species-dependent, it also varies with the tissue type. For example, most animal epithelial cells contain keratins, a diverse family of intermediate filaments consisting of more than 50 members, while mesenchymal and muscle cells are rich in the fibrous proteins, vimentin and desmin, respectively. Intermediate filaments found in neurons and glial cells include peripherin, neurofilaments, and glial fibrillary acidic protein (GFAP). A variety of associated proteins bind to intermediate filaments, either to improve stability (through crosslinking) or to provide attachment sites for other protein assemblies, such as actin filaments and microtubules.
Another highly specialized class of intermediate filaments are the nuclear lamins, which constitute the fibrous protein network that lines the inside of the nuclear membrane, as discussed above. Due to their close proximity to the membrane, nuclear lamins help attach the chromosomes to the nuclear membrane and provide anchorage points for the nuclear pores. Many scientists believe that nuclear lamins are the evolutionary ancestor of cytoplasmic intermediate filaments, which evolved through duplication and translocation of the gene product to the cytoplasm. The rigidity bestowed on cells by intermediate filament networks is especially useful to soft-bodied animals that do not possess an exoskeleton. Because intermediate filaments are very abundant in cells that are often subjected to high mechanical stress in vivo, it appears that their primary role is to provide physical strength to cells and tissues.
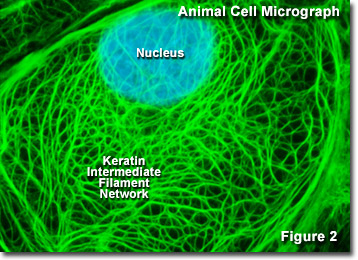
Presented in Figure 2 is a digital image of the keratin intermediate network found in a rat kangaroo (PtK2 line) epithelial cell as seen through a fluorescence optical microscope. The extensive intertwined network was labeled with primary antibodies to several cytokeratin proteins, which were then stained with secondary antibodies containing a green fluorescent dye. The nucleus was counterstained with a blue dye to note its location in relation to the intermediate filament network. Fluorescence microscopy is an important tool that scientists use to examine the structure and function of internal cellular organelles and the cytoskeleton.
As illustrated in Figure 1, intermediate filament monomer peptides are an elongated fibrous class of proteins with a central alpha-helical region capped with globular ends at both the amino and carboxylic acid termini. Two of the monomer units form a coiled-coil dimer that self-associates in an anti-parallel arrangement to form a staggered tetramer, which is the analogous soluble subunit for the globular actin monomer and the tubulin heterodimer (existing free in the cytoplasm). Tetramer units pack together laterally to form a sheet of eight parallel protofilaments that are supercoiled into a tight bundle. Each tightly coiled intermediate filament cross section reveals 32 individual alpha-helical peptides, which renders the filament easy to bend but quite difficult to break, thus accounting for the extreme structural rigidity. Although less is understood about the mechanism of intermediate filament assembly and disassembly, it is clear that some classes are highly dynamic structures with a significant rate of turnover in many cell types.
Mutations in intermediate filament genes lead to a host of rather uncommon diseases. For example, defective keratins in skin tissue lead to a disorder known as epidermolysis bullosa simplex, manifested by skin blisters produced with even a slight mechanical stress. Similar blistering diseases due to keratin mutations in other tissues affect the esophagus, eyes, and mouth. Several neurodegenerative diseases, such as amyotrophic lateral sclerosis (ALS or Lou Gehrig's Disease), are associated with malfunctions in the intermediate filament (neurofilament) network, and defects in desmin intermediate filaments produce muscle disorders.
