Photography of Beer and Cocktails
Through the Microscope
Although the following discussion is based on the art and science involved with photographing beer through the microscope, the same techniques also apply to mixed drinks and other cocktails, such as wine, liquor, and brandy. Beer is a complex and heterogeneous mixture that contains a wide variety of both low and high molecular weight carbohydrates, minerals, alcohol, trace amounts of proteins, yeast by-products, and other diverse organic compounds along with carbon dioxide in an approximately 10 percent weight to volume mixture with water.

Figure 1. Crystallites of frozen Budweiser
beer imaged on a microscope slide
with polarized light.
The composition of beer varies widely with brewing techniques but several variables remain fairly constant: water content (~90%), carbon dioxide saturation (~1/2% by weight), mineral content (usually trace quantities), and alcohol content (3-10% by volume). The biggest variable in commercial beers is the nature of their carbohydrate content, which varies widely with respect to concentration (usually between 30-75 grams per liter) and composition from beer to beer. There are also trace amounts of other polymers derived from the brewing process such as polyphenols and yeast by-products. This variation in the carbohydrate fingerprint of individual beers can be used as an advantage to produce a wide spectrum of crystallization motifs, as we will discuss below.
The key to obtaining good photomicrographs of beer is to apply microscopy methods that rely on contrast enhancement techniques to compensate for the lack of contrast commonly exhibited by beer samples. Polarized light microscopy can be used to capture the brilliantly colored crystalline patterns that arise through birefringence (double refraction) of the component materials in beer. Water, the chief component of beer, is birefringent in the frozen (ice) state and will produce colorful micrographs like the one illustrated in Figure 1. This photomicrograph was taken of frozen beer crystallites that were placed directly on a microscope slide and observed without a coverslip.
It is possible to capture many different beer photomicrographs using this simple freezing technique, the main problem being the overall similarity between the images. It confuses the general public when they are confronted by a large number of obviously related images and are told that these represent different beers. To circumvent this problem, additional approaches should be taken to generate different types of patterns for each beer in order to generate a large collection.
The second most convenient method for photographing beer is to atomize or convert the beer into a fine mist and to blast or spray the mist onto a smooth hydrophobic surface such as silicon, germanium, polystyrene, or treated glass. The photomicrograph in Figure 2 exhibits this technique when applied to the same Budweiser sample used to produce Figure 1. In this case the beer was chilled to 4° C, placed in a container and pressurized with Freon before being atomized and sprayed directly onto the surface of a highly polished silicon wafer.
The sample was imaged in a reflected light differential interference contrast (DIC) microscope using a quartz compensating plate between the Wollaston prisms and polarizers. DIC microscopy is a rather complicated technique, but it can be used effectively to produce dramatic and colorful images of both liquid and crystalline samples. The major drawback to DIC microscopy is the high cost of the equipment, which will generally add about $10,000.00 to the price of a polarized light microscope.
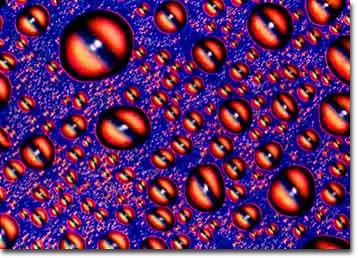
Figure 2. DIC photomicrograph of chilled Budweiser beer
sprayed onto a highly polished silicon surface and imaged
with a retardation plate.
It is possible to obtain images similar the one presented in Figure 2 with just about any beer but, as I discussed above, these will not help to differentiate between the different beer samples. A more useful technique is to freeze the beer with an apparatus designed to sandwich a few microliters of the beer sample between two glass plates and photograph the resulting birefringent crystallites in polarized light or with DIC microscopy. This procedure is very complex and involves the use of liquid nitrogen pumped into a specialized cryogenic cell to cool and freeze the sample.
A major problem with this technique is condensation of atmospheric water onto the surface of the glass plates, preventing the microscopist from being able to adequately focus and image the sample. High humidity conditions further aggravate the situation and, in many instances, the entire microscope must be encased in a plastic glove box with a nitrogen atmosphere. During conditions of low humidity (usually during the months of December through February in Tallahassee, Florida), it is sufficient to blow a stream of dry nitrogen over the surface of the sample to prevent condensation. Being able to keep the microscope out of a glove box makes the job of photographing samples through the microscope much easier.
Interesting effects can be obtained with the freezing cell if the sample is allowed to slowly heat up to room temperature. In this case, temperature gradients are established inside the cell causing the sample temperature to rise sporadically and create some interesting effects. We have been able to capture some very nice images using this technique. The main problem with this method is the lack of overall variation in the crystallite appearance from sample to sample. A typical photomicrograph of Budweiser prepared using this technique is illustrated in Figure 3. This image shows a temperature gradient that transverses from the top to the bottom of the photomicrograph and the changes induced when miniature crystallites of the beer start to melt.
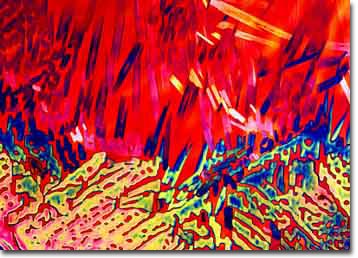
Figure 3. Budweiser beer crystallites captured
in the process of melting on a cryogenic
microscopy stage.
The top portion of the image shows faint crystallites of frozen beer that have almost completely melted, while the bottom portion shows a network of beer-ice crystallites that have started to melt but are still largely intact. The gradient across the cell is fairly sharp, and an apparent transition is occurring in the center of the image between a crystalline network and individual "icebergs" that will eventually melt.
Diverse effects can be obtained by varying the amount of dissolved carbon dioxide present in the beer when it is frozen. Carbon dioxide solubility in beer is inversely proportional to temperature so that freezing temperatures induce beer to release most of the dissolved carbon dioxide. By removing carbon dioxide from some samples with a mild vacuum, different types of effects can be obtained both with the cryogenic cell and using techniques that will be discussed below. Photomicrographs similar to the one presented in Figure 3 are commonly obtained with the cryogenic cell apparatus described above, however they show little genuine deviation from beer to beer.
The three techniques described above constituted our first approach to exploring photography of beer through the microscope. It rapidly became apparent that the combined methods would only allow us to generate about 7-10 unique photomicrographs and this would not be enough to generate the ~160 individually recognizable images necessary for either the Molecular Expressions BeerShots gallery or Stonehenge's necktie collections.
Our next approach borrows from techniques ("seed crystallization") commonly used in synthetic organic chemistry to prepare complex organic molecules. One of the most difficult problems in synthetic chemistry is to induce re-crystallization in dilute solutions of purified molecules that have been dissolved in a "poor" solvent to increase purity. In this case, the molecule of interest is partially soluble in the solvent, but impurities are very soluble and are removed.
Too often it is necessary to add large quantities of the solvent to get the target molecule dissolved and it will refuse to spontaneously initiate re-crystallization. To help matters, chemists often place a small "seed" crystallite into the solution to help initiate the crystallization process whereby the desired molecules will condense into solution crystals that can be collected by filtration. It is important to use only a small quantity of the seed crystallite to avoid contamination of the final sample.
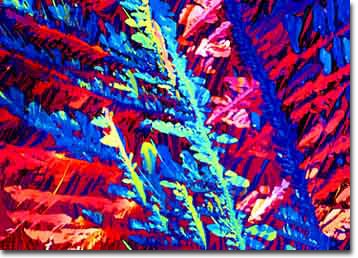
Figure 4. Budweiser beer crystallized using
the seed crystal technique to produce
very small crystallites.
When we first applied this technique to beer, mixed results were obtained and most of the experiments resulted in a failure to produce crystallites of sufficient quality for photomicrography. After many experiments, we decided to try sandwiching very small quantities of the seed crystals between a microscope slide and coverslip and applying beer to the void in the space where the seed crystallites had been planted. By allowing the beer to slowly evaporate (and often upon repeated application of new beer solution to replenish and concentrate the sample), we finally obtained nice crystallites of the beer carbohydrates through the use of seed crystals. A typical seed crystal-derived photomicrograph of Budweiser beer is illustrated in Figure 4.
Photomicrographs developed by this method allow for a wide variety of patterns to be obtained, a necessary factor in development of the BeerShots collection. Careful choices of the chemical and physical properties of the chemicals used as seed crystals can induce extreme variations in the crystalline motifs displayed by crystallized beer samples. For instance, by modulating the hydrophobicity (water-repelling ability) of the seed crystals, the average size of resulting beer crystallites can be increased (using less hydrophobic crystals) or decreased (more hydrophobic crystals). Likewise, the electronic properties and chemical structure of the seed crystal will also greatly influence the size and type of beer crystals formed through the beer crystallization process.

Figure 5. Large crystallites of Budweiser beer
obtained with seed crystals.
The photomicrograph presented in Figure 5 illustrates very large crystallites of Budweiser beer that were prepared using the seed crystallization technique with semi-hydrophobic seed crystals. This sample is an example of multiple re-crystallization where numerous aliquots of beer were applied to the seed crystal sandwich and allowed to slowly evaporate over a period of several weeks. A nice feature of this technique is that crystal size and geometry appear to be random and identical crystallization patterns are seldom observed with either the same or different beers.
This short overview of the photomicrography of beer crystallites only grazes the surface of a very complex, yet innovative approach to a new scientific art form. In theory, it is relatively easy to simply place a frozen beer sample in the microscope and obtain colorful images. In practice, this exercise is requires very sophisticated and expensive equipment, a detailed knowledge of photography and photomicrography, some experience in synthetic organic chemistry, and access to large library of seed crystals. The fact that extensive publication of these images over the past four years has yielded no serious competition testifies to the fact that the combination of techniques and skills required to perform complex sample preparation and photomicrography of beer are not common. Even granted the skill and equipment necessary to crystallize beer samples, success using this technique is also heavily dependent upon perseverance, intuition, patience, and a great deal of luck.
BACK TO SPECIALIZED TECHNIQUES
