Indole
|
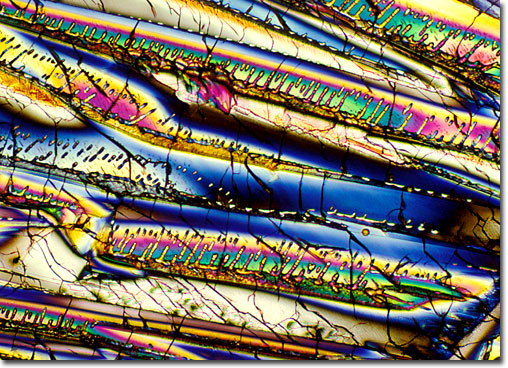
The indole nucleus, known to chemists as benzopyrrole, is the parent member of a broad spectrum of nitrogen heterocyclic biochemicals commonly found in nature. Indole derivatives occur in flower oils such as jasmine and orange blossom, and in less pleasant substances such as coal tar and fecal matter. Indoles also exist as melanin-related organics and indigoid pigments. One prominent indole configuration, indole-3-carbinol, has achieved notoriety as a therapeutic phytochemical. This recognition is not only because of the anticancer activity of indole-3-carbinol, but because the vegetables in which it occurs belong to the much maligned Brassica genus of cruciferous vegetables; the ever unpopular broccoli, brussel sprouts, cabbage, cauliflower, and kale. It would seem that indole-3-carbinol is partially responsible for the strong flavor that makes these vegetables so unpopular . . . and so healthy. Indole-3-carbinol is a highly effective anticancer agent, blocking carcinogenic substances before they reach their cellular targets and eliminating DNA damage in cell nuclei. It may also turn out to be an important chemical tool in fighting breast cancer because it inhibits estrogen-induced growth of cancer cells and converts the more dangerous forms of estrogen to safer forms. |
© 1995-2025 by Michael W. Davidson and The Florida State University. All Rights Reserved. No images, graphics, software, scripts, or applets may be reproduced or used in any manner without permission from the copyright holders. Use of this website means you agree to all of the Legal Terms and Conditions set forth by the owners.
This website is maintained by our
|