|
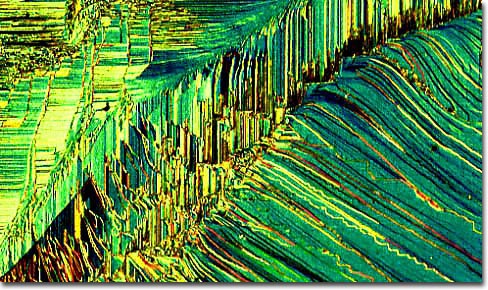
The superlattice shown above is comprised of the oxides of three different metals: iron, nickel, and magnesium. Both iron and nickel are lustrous, malleable, ductile, and silvery materials. Magnesium displays some similar characteristics to the other two metals, but is only malleable when heated and is highly reactive with water and various other materials. It has been used in pyrotechnics for many years because it burns a very bright white. Magnesium oxide, however, is often utilized in the production of synthetic rubber, cement, plastics, and refractory materials. The oxide of nickel, on the other hand, is often used in glass manufacture, ceramic glazes, and electroplating, while iron oxide is utilized for a wide range of purposes since it exists in three different forms (ferrous, ferric, and ferrosoferric).
|