|
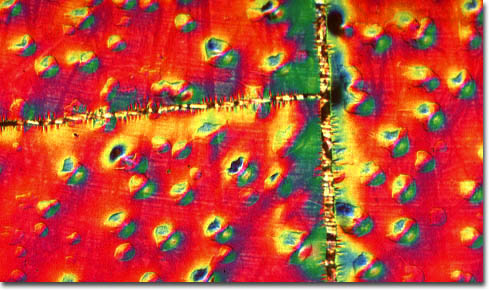
The metallic elements utilized to create the superlattice shown above are nickel and gold, which were deposited on a substrate of mica. Both nickel and gold are ductile, malleable, and beautifully lustrous materials that have a history of being utilized as forms of monetary exchange. Gold, however, was cherished by humans at a much earlier point in time than nickel, having been highly valued by various ancient civilizations, many of which used the metal to construct ornate decorative objects and jewelry. Nickel, on the other hand, was not isolated until 1751, when the Swedish Baron Axel Fredrik Cronstedt successfully extracted the material from an ore containing nickel arsenide, or niccolite. Nickel and gold both find a wide array of industrial use in modern times, often in the form of alloys. The chief industrial use of gold, which is highly conductive and inert, takes place in the electronics industry, where the substance is used, among other things, to plate contacts and terminals, as well as integrated circuits and other semiconductor systems. Nickel is a similarly popular material for plating, often serving as a protective covering of other metals.
|