|
Nucleotides are building blocks of the nucleic acids, perhaps the most fundamental and important constituents of the living cell. The nucleic acids were probably the first biomolecules to evolve and life could only begin with their evolution because they are the only biological substances that carry the potential for self-duplication. The blueprint for a living organism is encoded into its DNA or RNA (the two types of nucleic acids) and is passed on from generation to generation. The key to understanding how this important exchange of information takes place was discovered in the structure of DNA, which scientists James Watson and Francis Crick first described in 1953. DNA exists in the form of a double helix, two strands of the unusual molecule winding around one another into a spiral. Each strand, as Watson and Crick discovered, is like a very long piece of string consisting of monomer nucleotides, each comprised of a deoxyribose sugar, a phosphate group, and a nitrogenous base. The number of different nucleotides in DNA is limited because there are only four different bases in the nucleic acid (adenine, guanine, cytosine, and thymine), but variations in the ordering of the bases results in the tremendous amount of diversity found among living organisms.
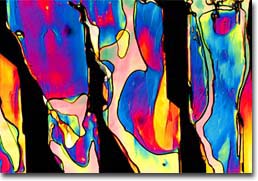
Cytidine
Similar to DNA, RNA is comprised of nucleotides, but the bases found in the organic molecule are slightly different than those in the other nucleic acid, uracil taking the place of thymine. Also, RNA nucleotides contain ribose sugars instead of deoxyribose. Ribose differs from deoxyribose by the presence of a hydroxyl group rather than a hydrogen atom attached to it 2' carbon atom. The number of phosphate groups contained in nucleotides may vary, but in both DNA and RNA only a single group is present per nucleotide. Each of these groups is linked to the sugar molecule of the adjacent nucleotide in the chain. It is also important to note that the nucleotides of one nucleic acid strand have a specific association with nucleotides in the corresponding strand. Due to the chemical affinity of the bases, in DNA nucleotides containing adenine are always paired with nucleotides containing thymine, and nucleotides containing cytosine are always paired with nucleotides containing guanine. In RNA, however, nucleotides containing uracil are paired with those that feature adenine. The complementary bases are linked to one another by weak chemical bonds known as hydrogen bonds.
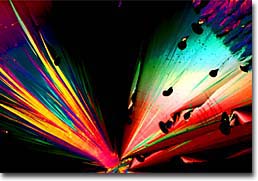
Thymidine
When a nitrogen base is connected to either a ribose or deoxyribose sugar molecule, the resulting compound is known as a nucleoside. It is only when one or more phosphate groups are added to such a compound that a nucleotide is formed. Generally, the phosphate is in ester linkage to the 5' carbon of the sugar molecule. If more than one phosphate is present, they typically exhibit acid anhydride linkages to one another. According to convention, the names of nucleosides depend upon whether they include a purine or pyrimidine base, those meeting the former condition ending in -osine (for example, adenosine and guanosine), while those conforming to the latter provision end in -idine (as in uridine, thymidine, and cytidine). The naming of nucleotides follows similar conventions, but also is designed to reflect whether the biomolecules contain one, two, or three phosphate groups. For example, adenosine triphosphate (ATP), which appears in the banner photomicrograph of this collection, is a nucleotide with three phosphate groups. Though not shown here, adenosine monophosphate (AMP) and adenosine diphosphate (ADP) are other common nucleotides.

Uridine
Nucleotides are widely available in foods and easily obtained in the diet, but there are also biosynthetic pathways and salvage processes that ensure a complete balance of these important biochemicals. In addition to heredity, nucleotides are also involved in a number of intermediary metabolism biochemical reactions that have little or nothing to do with the storage of genetic information. ATP, for example, is heavily involved in most biosynthetic pathways as an important energy-storage and transfer molecule. Other purine and pyrimidine nucleotides and analogs are also involved in important biochemical reactions. Adenosine is coupled to a nicotinamide analog nucleotide to produce NAD and NADP, two coenzymes that are used quite frequently in electron-transfer biochemical mechanisms. Likewise, adenosine is also a part of the Coenzyme A molecule, an acetyl transfer reagent, and cobalamine (vitamin B-12), a methyl group donor to biochemical reactions. Other notable functions of nucleotides include their ability to act as structural substituents for important coenzymes and vitamins, and as metabolic regulators and signal molecules, making the substances a very important class of biomolecules.
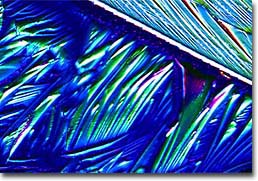
Guanosine
The scientific development of synthetic nucleotide analogs was well underway in the mid-1900s, and today many of the substances are utilized therapeutically. A leading pioneer in the field was British biochemist Alexander Robertus Todd, who was knighted in 1954 and received the 1957 Nobel Prize for Chemistry based upon his research on the structure and synthesis of nucleotides, nucleotide coenzymes, and nucleosides. One of the primary areas that nucleotide analogs have been used in the medical field thus far is in the treatment of cancer. The substances are well suited for such an application because they have the ability to interfere with DNA synthesis and, therefore, target rapidly dividing cells such as tumor cells. A few of the nucleotide analogs currently included in chemotherapy treatments include fluorouracil, mercaptopurine, and thioguanine. Other nucleotide analogues, such as azidothymidine (AZT) and dideoxyinosine (DDI), have been found useful as therapeutics for human immunodeficiency virus (HIV).
|




