The Fatty Acid Collection

Oleic Acid
Fatty Acids
|
Important constituents of lipids, fatty acid molecules characteristically consist of an even number of carbon atoms linked in a chain bonded with hydrogen atoms that features a carboxyl group at one end. When all of the carbon-to-carbon bonds that hold the chain together are single, the fatty acid is said to be saturated. However, if one or more of the bonds is double, the molecule is unsaturated. As implied by their name, monounsaturated fatty acids only exhibit one double bond, while polyunsaturated acids possess two or more. A few fatty acids that contain triple bonds are also known to exist, although they are far less common than other fatty acids. 
Lauric Acid Although lipids and fatty acids are relatively large molecules, they are not biopolymers made up of consecutive units like their cousins DNA, RNA, proteins and polysaccharides. Fatty acids have two chemically different structures combined to allow the unusual properties necessary for these biochemicals to perform their biochemical functions. The generalized structure for fatty acids contains a hydrophilic (water-loving) acidic "head" and a hydrophobic (water-fearing) hydrocarbon "tail". This structural combination is similar to that found in common soaps and fatty acids display a number of soap-like properties. Due to concerns about cholesterol and heart disease, scientists have been very interested in determining which types of fats and oils are best for one's health. All such lipids contain mixtures of fatty acids, but are designated saturated, monounsaturated, or polyunsaturated based upon their predominant component. Fats chiefly consisting of saturated fatty acids are quite stable and are typically found in solid form at room temperature. Studies have shown that these fats may raise blood cholesterol and can contribute to an increased risk of heart disease. Monosaturated fats, which are usually liquid oils at room temperature but may begin to solidify if exposed to colder environments, have often been found to be less unhealthy that saturated varieties. Polyunsaturated fats, however, are an even greater improvement over saturated fats, and substituting these oils, which remain in liquid form even when placed in a refrigerator, for solid saturated varieties may help lower total blood cholesterol. 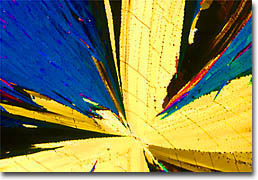
Oleic Acid Making the issue of "good" versus "bad" fats an even more complex issue, however, is the common process of hydrogenating vegetable and fish oils. In nature, unsaturated fatty acids are typically found in the cis form, in which their hydrogen atoms are located on the same side of their double carbon bonds. Yet, when lipids are hydrogenated, hydrogen atoms can be found on opposite sides of the double bonds they contain, forming what are known as trans fatty acids. In order to extend the shelf life of various food items or to create a solid product, such as margarine, from polyunsaturated oils, which may become rancid rather quickly in their unaltered form, companies often hydrogenate the fatty acids they contain. Though these products were originally believed to be better for the health of consumers than if they contained saturated fats, little evidence remains to support this notion. Most recent studies have suggested that trans fatty acids raise low-density lipoprotein (LDL) and total blood cholesterol levels, while reducing beneficial high-density lipoprotein (HDL) levels. 
Docosahexaenoic Acid Fatty acids perform a variety of biochemical functions in the human body, ranging from aiding in the maintenance of the immune system to facilitating the development of healthy cell membranes and enabling the production of prostaglandins, thromboxanes, and other eicosanoids, which are involved in the regulation of vasoconstriction, blood viscosity, blood pressure, and similar activities. However, humans, as well as many other animals, are unable to synthesize all of the fatty acids they need, and some must be obtained from the diet. These essential polyunsaturated fatty acids generally consist of linoleic and alpha-linolenic acids, but arachidonic acid is sometimes also included in the group although it may be synthesized from linolenic acids. Some good dietary sources of linoleic acid, which is part of the Omega-6 family, are green leafy vegetables, nuts, grains, seeds, and the oils made from them. A constituent of the Omega-3 group, alpha-linolenic acid is found in considerable quantities in similar items and is especially prevalent in flaxseed and fish oils. The human body is able to metabolize fatty acids through the progressive division of pairs of carbon atoms, which are then converted into acetyl coenzyme A. This coenzyme is oxidized as part of the citric acid cycle that is also involved in the breakdown of the sugar glucose. Through this process, fatty acids yield relatively large amounts of adenosine triphosphate (ATP), making the molecules an excellent source of energy, a fact that is supported by the tendency of the human body to store excess fuel as fat. Nevertheless, some parts of the body, such as the brain, are unable to utilize fatty acids as an energy source and must instead depend upon glucose metabolism to support their activity. 
Linoleic Acid The Molecular Expressions collection of fatty acids contains only a few members of this important class of biochemicals. Fatty acids are very difficult to crystallize and this makes it difficult to obtain high-quality photomicrographs. Also, they have been hard for us to obtain, and we are looking for new fatty acid and lipid samples. Should you be able to provide us with any samples in this arena please contact us by phone or e-mail. |
© 1995-2025 by Michael W. Davidson and The Florida State University. All Rights Reserved. No images, graphics, software, scripts, or applets may be reproduced or used in any manner without permission from the copyright holders. Use of this website means you agree to all of the Legal Terms and Conditions set forth by the owners.
This website is maintained by our
|