MOON ROCKS UNDER THE MICROSCOPE
Michael W. Davidson
NHMFL and MARTECH
The Florida State University
Tallahassee, Florida USA
INTRODUCTION
In the late 1600s, Galileo was the first to examine the Moon's surface with a telescope, and in the 300 or so years since, we have learned a great deal about the properties of our nearest neighbor. Without leaving our planet we have determined the Moon's size, shape, and weight, how to measure the temperature of the surface, and to estimate various electrical properties from radar waves reflected from the surface (1). However, investigators were not able to get a real handle on the geology of the Moon until mineral and soil samples were returned to Earth for close scrutiny.
|
|
|
Between 1969 and 1972 the National Aeronautics and Space Administration (NASA) successfully landed 12 Apollo astronauts on the lunar surface. The astronauts who visited the Moon carefully collected 2, 196 documented samples of lunar soils and rocks weighing a total of 382 kilograms during approximately 80 hours of exploration (2). It is important to note that these samples were gathered from a harsh lunar environment that included wildly fluctuating temperatures in an almost complete vacuum, potentially dangerous solar irradiation, and the uncertainty of return to Earth due to equipment failure.
Geologists hoped that exploration of the lunar surface would establish its composition, internal structure, geological history, and evolution. In this respect it was thought that the Moon would serve as a model for the early history of the Earth and the rest of the solar system. Yet, from the Apollo missions, it has been determined that the Moon is far more complex than previously thought. The Earth's only satellite, which was formed approximately 4.6 billion years ago along with the rest of the planets, has completely melted and differentiated twice in its history. The first melting occurred very shortly after initial formation of the Moon and apparently resulted in a widespread molten ocean that cooled to form a thick crust. While the satellite was cooling to form a solid body, several sections of the interior remelted, presumably due to radioactive isotopic decay, to form lava flows on the side facing the Earth (1-3).
|
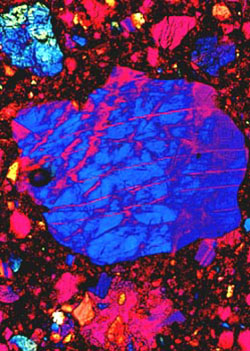
Transmitted polarized light photomicrograph of lunar basaltic lava sample collected from the Oceanus Procellarum mare region by Apollo 12 astronauts. This sample is 3.15 to 3.35 billion years old and gave the first indications that Granite exists on the moon. |
STORAGE AND PREPARATION OF LUNAR SAMPLES
The lunar samples were stored in sealed "rock boxes" during the return to Earth, although some samples were exposed to the atmosphere of the Lunar Landing Module and the Command Module. After arriving at the Johnson Space Center in Houston, Texas, all lunar samples were quarantined for 6 weeks in the Lunar Receiving Laboratory to insure the absence of extraterrestrial life forms (2). During the quarantine period, the samples were cataloged, photographed, and given a preliminary examination. After more careful examination, it became evident that life forms, organic molecules, and water was absent from the lunar samples and the quarantine was discontinued for the later Apollo missions.
Lunar samples are stored and prepared for allocation to research scientists in the Lunar Curatorial Facility at the Johnson Space Center. This facility provides a high-efficiency air filtration system that serves to remove dust and other particles from the laboratory air to a degree of less than 1000 particles per cubic foot. A slightly positive air pressure is maintained within the building and the floor plan restricts access to the storage vaults so that areas with the most traffic are separated from areas where the samples are kept.

|
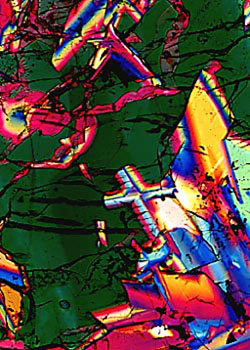
Transmitted polarized light photomicrograph of a lunar impact breccia sample collected from the Descartes highland plains region by Apollo 16 astronauts. This sample is 3.8 to 4.2 billion years old and gave evidence that the lunar highland plains were formed by meteorite impacts. |
The samples are stored and examined exclusively in nitrogen-filled glove boxes. Only ultra high purity nitrogen gas, produced by boiloff of liquid nitrogen, is used to store the rocks. This somewhat inert atmosphere prevents chemical changes in the samples that would be unavoidable in the open atmosphere. The most severe reaction would probably be oxidation of iron grains in the samples. The lunar samples are only allowed to come into contact with three materials: aluminum, teflon, and stainless steel. Investigators handling the rocks use teflon overgloves and stainless steel tongs. These tools are periodically cleaned with acid and rinsed in freon to avoid any cross-contamination of the samples. Contamination by organic substances in continuously monitored with ultraviolet lamps. During storage, the samples are kept in sealed teflon bags that are placed into stainless steel containers with bolt-on tops. A positive seal is provided by an aluminum gasket between knife edges on the top and bottom of the container lid. The sample containers are permanently stored in nitrogen-filled cabinets in a bank-like vault with thick steel-reinforced concrete walls.
Lunar samples probably serve as their own best containers so they are not cut up unnecessarily in an effort to avoid contamination (2). When a sample is needed for an investigation, it is transferred into a glove box and removed from its sealed container. A rigid protocol, designed to minimize contamination, is used for removing the various layers of teflon bags. The glove boxes are cleaned with liquid freon between samples to prevent cross-contamination from sample dust, and only one parent sample is allowed to occupy a glove box at any particular time. Also, separate glove boxes are used for each Apollo mission.
The procedure for processing lunar samples involves carefully weighing, photographing, and describing each individual sample. As the rocks are cut apart, each subsample is also weighed and photographed. The photographs all contain the image of an orientation cube that relates the orientation of the subsample to that of the parent and also to the original lunar surface orientation. This is essential so that orientation parameters are available for solar flare, cosmic ray and micrometeorite exposure studies. During the cutting process, the samples are dusted with nitrogen gas. Maps are made of the saw cuts and the resulting surfaces of the rocks using binocular microscopes. The large rocks are initially cut apart with a diamond-edged bandsaw inside the nitrogen filled glove box. Since it is impractical to cool the surface of the rock during this cutting procedure, some rocks probably grow quite hot during this process. A certain degree of contamination is probably introduced at this point because a metal smear can be observed on the sawn surfaces of some of the hardest rocks. The resulting slabs from large rocks and smaller samples are broken up with a stainless steel chisel. Immediately after completion of the subdividing operation, the subsamples are reassembled into their original positions and photographed as a group for the purposes of documentation. The photographs are used to construct three-dimensional maps that locate any particular subsample so that investigators will be aware of the position of their samples in relation to others that might appear in the scientific literature.
To prepare thin sections for microscopy (2, 3), a sample is first impregnated with epoxy under vacuum. Next, the sample is cut with a diamond blade using alcohol as a coolant and then ground flat with silicon carbide followed by polishing with diamond. After polishing, the sample is affixed to a glass microscope slide with epoxy resin. The final preparation involves grinding the sample to an approximate thickness of 100 microns followed by hand polishing to a 30 micron thickness using diamond paste on bond paper. Absolute ethyl alcohol is used in all phases of this preparation to avoid unwanted hydration of the samples.
|
Transmitted polarized light photomicrograph of a regolith breccia consisting of a wide variety of glass, mineral, and lithic fragments in a brown glass matrix. It was recovered on the slope of the Apennine Front at the Apollo 15 landing site. |
Examination of thin sections prepared from lunar samples is best done with transmitted polarized light (see Figures 1-5). Using this technique, investigators can get a handle on many of the properties displayed by these samples. Characteristics such as twinning, melt inclusions, cracks, fractures, and zoning can be readily documented with polarized light microscopy. In addition, the birefringence of the samples can be recorded with quarter-wavelength and one wavelength retardation compensators. Many samples are quite colorful under polarized light and color photomicrography can yield beautifully colored micrographs (Figures 1-4). Reflected light microscopy also aids in the identification of minerals present in the samples and can be used to the anisotropy and reflectivity of the samples.
All photomicrographs of lunar samples described in this report were recorded on a Nikon Optiphot-Pol transmitted polarized light microscope equipped with a UFX-III in-camera photomultiplier system (4). Images were recorded on Fujichrome 64T and were usually underexposed 1-3 f-steps by adjusting the ISO setting on the exposure monitor. Kodak E-6 transparency reversal photographic processing was chemically modified to enhance contrast and color saturation. In addition, processing times were extended 30-45% in the first developer to "push" greater contrast into the images (3).
EXAMINATION OF THE LUNAR SAMPLES
The lunar surface can be roughly divided into two domains. About 20% of the Moon's surface is covered by dark lunar mares (Latin for seas) or lowland regions. At a low sun angle, the lunar mares display wrinkle ridges and flow fronts indicating that they are filled with frozen liquid. Before the Apollo missions, it was thought that the mares were relatively young because they are so poorly cratered (1, 2). The Apollo astronauts recovered a large collection of samples from the mares which, to the surprise of many geologists, gave evidence that the maria are extremely old with ages ranging from 3.1 to 3.8 billion years. The mares are filled with basaltic lava similar to the lavas found here on Earth. Mare basalts are volcanic lavas generally rich in iron and titanium oxide minerals that formed when molten rock from the interior of the Moon surfaced and cooled. For chemical purposes, the mare basalts can be divided into two groups (2). An older high titanium group with ages ranging from 3.5 to 3.8 billion years was collected from the Mare Tranquillitatis and Taurus-Littrow mare regions by Apollo 11 and 17 astronauts, a sample of which is illustrated in the polarized photomicrograph displayed in Figure 1.
|
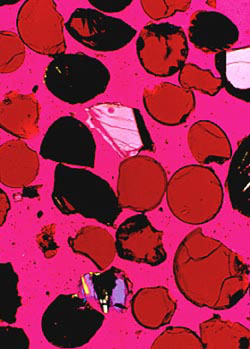
An unusual orange soil collected near a small crater at the Apollo 17 landing site. The sample is composed primarily of small orange glass spheres, and black devitrified (non-birefringent) spheres. The sample also contains a small amount of fragmental debris derived from mare basalts. This glass sample is approximately 3.48 billion years old. |
This basalt sample is rich in iron and titanium and gave important first clues as to a lack of water on the moon and proved that the lunar maria are very old. A younger, low titanium, group of basalts with ages ranging from 3.1 to 3.4 billion years was recovered by the Apollo 12 and 15 astronauts. These age differences and the wide variety of different mare chemistries, where the titanium oxide composition varies from 1 to 13 percent, indicates that the mare basalts could not have been generated from one common source region or common parental magma through different degrees of partial melting. Figure 2 is a low magnification polarized transmitted light photomicrograph of a polished thin section (30 m) cut from a sample collected from the Oceanus Procellarum mare region by Apollo 12 astronauts. Studies conducted on this sample (5) indicate that this is a low titanium, medium-grained, olivine basalt that possesses an exceptionally high magnesium content. Large pyroxene oikocrysts surround numerous rounded olivine crystallites, and patches of glomerophric olivine are present in some areas. The sample is composed primarily of the minerals olivine, plagioclase and pyroxene with a trace amount of opaques. Chemically, the rock is about 42% silicon dioxide, 22% ferrous oxide, with the rest being mainly magnesium, calcium, and aluminum oxides (2).
Surrounding the mares are the lighter-colored hilly and mountainous highlands. The rugged highlands are covered with countless small craters formed when meteorites struck the surface early in the history of the Moon. The lunar mares also possess craters, although not as many because the rate at which meteorites hit the Moon has decreased with time. Most of the rocks found in the highlands are breccias, composed of lithified aggregates of clastic debris and melt produced by meteorite bombardment of the lunar surface. Most of the breccias were recovered by the Apollo 14 and 16 missions to the Fra Mauro and Descartes plains regions in the lunar highlands. These samples proved to be very old with ages ranging from 3.9 to 4.0 billion years. Lunar breccias have a wide variety of matrix textures ranging from fragmental to vitric to crystalline. Figure 3 is a high magnification polarized transmitted light photomicrograph of an impact-melt breccia that was collected from the lunar highlands during the Apollo 16 mission. As can be seen in the micrograph, it is a very dense and coherent sample with a distinctive poikilitic texture. This sample has been studied in detail by Albee and co-workers (6), and has been radioactivity-dated at about 3.93 billion years.
The lunar surface is covered by a layer of disorganized debris which has been termed the lunar regolith (2). The thickness of this regolith varies from about 5 meters in the mare regions to approximately 10 meters in the lunar highlands. A majority of the regolith is composed of a fine gray soil with a density of about 1.5g/cm3, however the regolith also contains breccias and fragments from the local bedrock. Figure 4 is a transmitted polarized light photomicrograph of a lunar regolith breccia recovered part way-up the slope of the Apennine front by Apollo 15 astronauts. The sample was studied in detail by Simon and his colleagues (7) and has been found to contain a wide variety of glass, mineral, and lithic fragments encapsulated into a brown glass matrix.
|
A microscape fabricated with lunar samples. The foreground is a basaltic lava sample recovered by Apollo 12 astronauts. The cloudy sky is an epoxy resin bead defocused with a 530nm retardation plate and a blue filter inserted partially into the light path. The "sun" is the image of the field diaphragm partially defocused through a red filter and moved slightly off center. |
An unusual lunar sample was collected from an orange soil near a small crater at the Apollo 17 site (2). This soil sample is thought to originate from the "dark mantle" that covers a wide area around this lunar landing site. Figure 5 depicts a photomicrograph of the sample showing a composition of small orange glass spheres, fragments of spheres, and black devitrified spheres. The sample also contains a small amount of mixed fragmental debris from mare basalts. The glass in this sample was probably from either lava fountaining, vapor condensation following a meteorite impact, or from a meteorite impact into a liquid lava lake. Most lunar scientists now accept the lava fountain hypothesis because trace element data do not support a meteorite origin.
Most of the minerals found in the Moon rocks had already been examined and characterized during routine geological investigations of rocks found here on the Earth. The exception was three minerals that could only have been produced under the extreme conditions found on the Moon. A new mineral rich in titanium and iron oxides was named armalcolite after Neil Armstrong, Edwin Aldrin, and Michael Collins, crew members of Apollo 11, the first mission to successfully land on the Moon. Rocks found in the lunar highlands are generally rich in the common white mineral feldspar that is composed of calcium and aluminum silicates. A highly unusual class of rocks found in the highlands was given the nickname KREEP (1, 2) after its unique composition of potassium (K), rare-earth elements (REE), and phosphorus (P).
The age of the lunar samples was determined by the abundance ratio of radioactive isotopes (2). It has been found that the age of most highland rocks has been reset by shock and heat produced by huge meteorite impacts. From radioactivity dating, researchers can determine when these impacts occurred. Most of the samples have ages centering around 4.0 billion years, although some rocks have escaped meteoritic bombardment and are as old as the Moon itself (4.6 billion years).
In spite of all that has been learned from examination of lunar samples, there is yet many unanswered questions about the Moon. For instance, the most fundamental question is still how the Moon was formed. Theories range from formation with the Earth as a double-planet system, fission from the Earth followed by establishment of a new orbit, to capture by the Earth as the Moon traveled through space. Another central question is the asymmetry of the Moon. The side facing the earth is filled with the dark mare regions while the opposite side is composed mostly of highlands. What influence did the presence of the Earth have on this phenomena? These and many other questions remain unanswered until more research can be conducted on the lunar samples and on the Moon itself. For a complete accounting, it may be necessary to venture back to the surface of our nearest neighbor.
CREATIVE PHOTOMICROGRAPHY
Photography is slowly becoming recognized as a legitimate art form and it's increasing popularity is evidenced by the abundant use of photomicrographs as "eye-catchers" on the front covers of books and periodicals (8, 9). In this respect, I have introduced a series of multiple-exposure photomicrographs, termed microscapes (4, 9, 10), that are intended to resemble surrealistic imaginary landscapes. Figure 6 is a microscape entitled "Baghdad After the Storm", constructed with Apollo 12 lunar samples. The foreground is a single exposure of a basaltic lava sample collected from the Oceanus Procellarum mare region by Apollo 12 astronauts and is intended to resemble rubble from destroyed buildings. After masking the lunar sample exposure by covering a portion of the field lens, a second exposure is made with a defocused epoxy resin bead and a 530 nanometer retardation plate (the red cloudy sky). The rising sun effect is generated by reducing the aperture of the field diaphragm and slightly decentering the substage condenser. After defocusing the shutter leaves, a red filter is placed in the light path and a third exposure is made. The final result, as shown in Figure 6, resembles a hauntingly realistic image. With careful experimentation, a wide spectrum of photomicrographs of this type can be assembled, limited only by the individual photomicrographer's imagination.
Author's Biography
Michael W. Davidson is a research scientist in charge of the optical and scanning probe microscopy facilities at the National High Magnetic Field Laboratory at The Florida State University. His numerous interests include the packaging of DNA into virus heads, liquid crystallinity in biological systems, and the interaction of drug molecules with DNA. He has published numerous scientific articles in these fields. His photomicrography (photographs taken through a microscope) has appeared on the covers of over 200 scientific and trade periodicals and books and he has written more than 50 articles on the subject in popular photography magazines throughout the world. In addition, he has won over 30 awards in scientific and industrial photography competitions. Framed prints of Davidson's photographs have been exhibited at the American Association for the Advancement of Science and the National Research Council Headquarters in Washington, D.C., the International Center for Photography and Sotheby's in New York City, and the Los Angeles Municipal Art Gallery. Most recently, Davidson's photomicrographs have appeared on greeting cards, gift-wrap paper, T-shirts, trading cards, and calenders.
ACKNOWLEDGEMENTS
The author would like to thank the National High Magnetic Field Laboratory (NHMFL) for continued support, and Drs. Charles Meyer, John Dietrich, and James Gooding of the Johnson Space Center for helpful conversations and providing the lunar samples. Microscopes used in this study were provided by the Nikon Instrument Group, Melville, NY.