
Nickel-Hydrogen Batteries
The nickel-hydrogen battery is a sealed secondary battery, and combines the technologies of batteries and fuel cells.
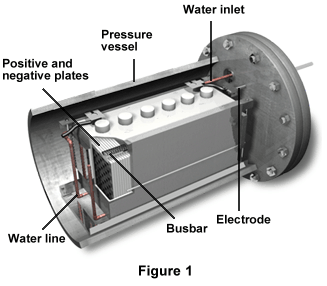
Figure 1 displays a 6-volt 100-Ah terrestrial nickel-hydrogen battery. The cutout portion shows the various module components. The positive and negative plates attach to the busbar, and the battery contains a water line, a water inlet, and a pressure vessel as well.
ChemistryThe nickel-hydrogen battery has a nickel oxide positive electrode similar to the nickel-cadmium cell, and is like the hydrogen-oxygen fuel cell since it has a hydrogen negative electrode. This hybrid battery has a long cycle life, high specific energy, high power density, and also exhibits tolerance for overcharge, and is therefore the choice battery in many aerospace applications, especially geo-synchronous (GEO) and low earth-orbit (LEO) satellites. In addition, the battery's hydrogen pressure is a good indicator of the charge state of the battery. Recently, nickel-hydrogen batteries have also been used in terrestrial applications. Its disadvantages include an expensive initial cost, as well as low volumetric energy density.
The positive electrode reactions happen similarly to those in the nickel-cadmium system. Hydrogen gas in the negative electrode becomes oxidized to water at discharge, only to be reformed at charge via electrolysis. Oxygen is formed at the positive electrode at overcharge, and there is no alteration of the potassium hydroxide (KOH) or water level in the battery during continuous overcharge. The positive electrode makes hydrogen during reversal, which in turn is consumed at the same rate at the negative electrode. In addition, hydrogen reacts electrochemically but not chemically, and reduces the nickel oxyhydroxide.
The sintered positive electrode is made up of a sintered porous nickel plaque, which contains active material of nickel hydroxide. The plaque conducts the battery's electric current, and retains the active material. An electrochemical process, either the aqueous impregnation process or the alcoholic impregnation process, forces the active material into the sintered plaque. Both processes load the active material uniformly within the pores of the nickel sinter, and also control the loading level.
The hydrogen electrodes utilize a teflon-bonded platinum black catalyst. The battery can use two types of separators in aerospace cells: fuel-grade cell asbestos paper and untreated knit Zircar cloth. These types of separators keep the positive and negative electrode from contacting each other, remain stable in the electrolyte (which permits long-term storage and cycling), and act as a medium for charge and discharge current.
When in orbit, satellites go through periods of time called eclipse periods, where the satellite itself is in the shadow of the Earth. The peak of the periods happens at the equinoxes, about March 21 or September 23, and the seasons last about 45 days. With GEO applications, communications satellites, which use nickel-hydrogen batteries, must operate normally and without interruptions during eclipse seasons. The eclipse periods, when the satellites are in the Earth's shadow, can be anywhere from a few minutes to seventy-two minutes in duration. During summer and winter solstices, the batteries are charged on "trickle charge." In LEO applications, the satellite's orbit is 96 minutes, and must take into account the eclipse periods when charging and discharging.