
The Alkaline-Manganese Battery
The alkaline battery gets its name because it has an alkaline electrolyte of potassium hydroxide.
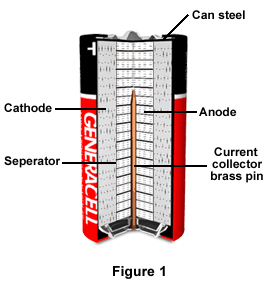
As illustrated in Figure 1, the battery’s container is a cylindrical steel can, and serves as a current collector. The cathode of manganese dioxide and carbon is a hollow cylinder within the can, located very close to the can surface. Inside the hollow cathode are separator rings, which are positioned in layers, and inside the separator rings is the anode. The battery has either a metal or plastic jacket, as well as covers on its bottom and top, to help control polarity in applications.
General InformationThe alkaline-manganese dioxide battery was introduced in the early 1960’s and remains in a strong position in today’s battery market. Theoretically, alkaline-manganese dioxide cells have a higher capacity than Leclanche, or zinc-carbon cells, of similar sizes. This is due to higher purity and activity of the manganese dioxide, the dense cathode with little electrolyte, and the efficient spacing of components. The voltage for this battery begins above 1.5 volts, which decreases gradually during discharge. In addition, the battery can function at temperatures up to 55 degrees Celsius.
Evolution of Today’s Alkaline-Manganese Dioxide BatteryThe alkaline system has many advantages over the competing Leclanche or zinc-carbon battery. Alkaline batteries come in two forms, either as large cylindrical cells, or as miniature cells resembling buttons. Since its beginnings, the alkaline system has gone through many changes. First, it began using a gelled and amalgamated zinc powder anode within a compartment in its center while at the same time employing vented plastic seals. A butt-seam metal finish allowed the battery better internal volume. Furthermore, the use of organic inhibitors helped bulge and leakage problems, and a reduction of mercury helped make the battery even more efficient and environmentally sound. Today's version is 60% more powerful than the original alkaline battery that was introduced in the early 1960’s.
ChemistryThe alkaline-manganese dioxide battery contains electrolytically manufactured manganese dioxide and aqueous alkaline electrolyte, as well as zinc metal as a powder. Electrolytic manganese dioxide is more pure than standard reagent manganese dioxide, and has a greater reactivity. The electrolyte is caustic, and therefore, reduces the hydrogen gassing rate.
The cathode is comprised of a manganese dioxide and carbon mixture. Binders may also be added, as well as an electrolyte solution or water, to help build the cathode. The manganese dioxide causes the oxidation. Carbon is used in the cathode because manganese dioxide is not a good conductor on its own. Often, the carbon is in a graphite or acetylene black form, and the anode contains zinc powder, which is very pure due to distilling or electroplating from an aqueous solution. The powder is formed by atomization. The gelling agents are usually starch, polyacrylates, or ethylene maleic anhydride copolymers. The separators must be ionically conductive while at the same time insulating, and must be stable during oxidation and reduction. The battery’s container does not assist discharge in any way, and is usually made of steel.