Polarized Light Microscopy Digital Image Gallery
Uridine
Uracil is an organic base that was first isolated and synthesized in 1901. When this member of the pyrimidine family is combined with a ribose ring via a glycosidic bond, a nucleoside called uridine is formed, which can be phosphorylated to produce one of three nucleotides: uridine monophosphate (UMP), uridine diphosphate (UDP), and uridine triphosphate (UTP).
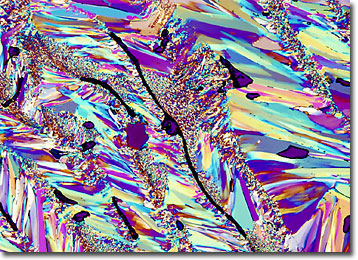
Uridine is one of four nucleosides used in genetic coding for RNA, and its complement is the nucleoside adenosine. Each molecule of uridine is comprised of 9 carbons, 12 hydrogens, 2 nitrogens, and 6 oxygen atoms, resulting in a molecular weight of 244.20. The melting point of purified crystalline needles of uridine, which are soluble in water, is 165 degrees Celsius. Within the body, the nucleoside plays an important role in the metabolism of carbohydrates, and, in the laboratory, the white odorless powder that can be extracted from yeast ribonucleic acids using a weak alkali solution is utilized in a variety of biochemical experiments and studies.
Due to their therapeutic capacity, a number of nucleotide analogues are often chemically synthesized for medicinal use. When certain uridine derivatives are, for instance, utilized with organ transplant procedures, the likelihood of organ rejection by the recipient is reduced due to immune system repression. Also, mitochondrial disease, which is typically associated with neurological problems and a decrease in cognitive ability as well as kidney and muscular malfunction, may be potentially improved when triacetyluridine (TAU) is administered to sufferers. TAU is a precursor of uridine that rapidly converts to the nucleoside when consumed orally.
