Fluorescence Digital Image Gallery
Horse Dermal Fibroblast Cells (NBL-6)
|
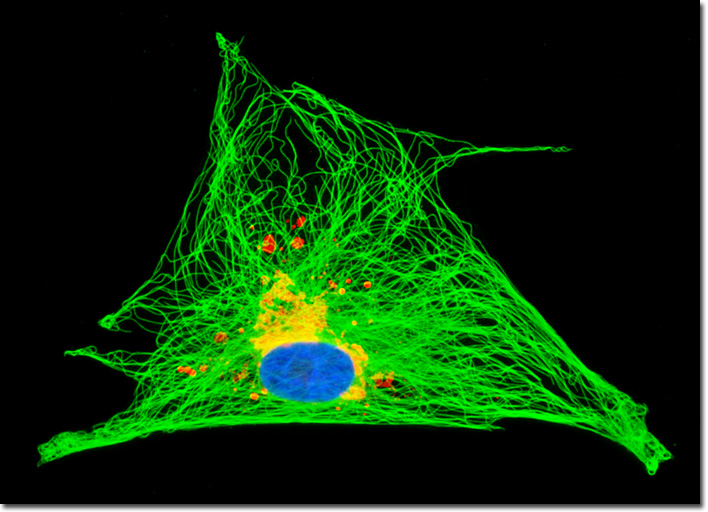
Tubulin is a protein that comprises the basic structural subunits of microtubules, the cytoskeletal filaments associated with the positioning of membrane-bound organelles and intracellular transport. Each tubulin subunit is, in fact, a heterodimer composed of alpha-tubulin and beta-tubulin components linked to one another by noncovalent bonds. The two globular proteins, which only occur in this network, each exhibits a binding site for a single guanosine triphosphate (GTP) molecule. The GTP molecule bound to the alpha-tubulin monomer may be regarded as a fundamental component of the tubulin heterodimer complex because it is physically unable to be exchanged or hydrolyzed, instead remaining constantly confined at the dimer interface. The nucleotide in the beta-tubulin monomer, however, is exchangeable and may be hydrolyzed to produce guanosine diphosphate (GDP), an event that significantly impacts the dynamics of the microtubular network. In order to visualize the relationship between the Golgi complex and the intracellular microtubular network, the culture of horse dermal fibroblast (NBL-6) cells presented in the digital image above was immunofluorescently labeled with anti-tubulin (pan) mouse monoclonal primary antibodies followed by goat anti-mouse Fab fragments conjugated to Alexa Fluor 488. Golgi bodies were simultaneously targeted with rabbit anti-giantin primary antibodies, followed by goat anti-rabbit secondaries conjugated to Alexa Fluor 568. Nuclei were counterstained with Hoechst 33342. Images were recorded in grayscale with a QImaging Retiga Fast-EXi camera system coupled to an Olympus BX-51 microscope equipped with bandpass emission fluorescence filter optical blocks provided by Omega Optical. During the processing stage, individual image channels were pseudocolored with RGB values corresponding to each of the fluorophore emission spectral profiles. |
© 1995-2022 by Michael W. Davidson and The Florida State University. All Rights Reserved. No images, graphics, software, scripts, or applets may be reproduced or used in any manner without permission from the copyright holders. Use of this website means you agree to all of the Legal Terms and Conditions set forth by the owners.
This website is maintained by our
|