Fluorescence Digital Image Gallery
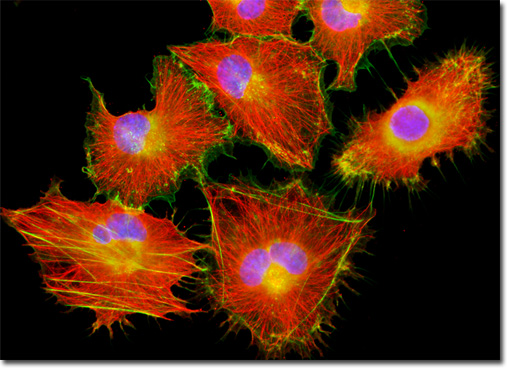
Baby Hamster Kidney Fibroblast Cells (BHK-21)
|
Phalloidin is one of the two main toxins produced by the large, poisonous death cap mushroom (Amanita phalloides). First differentiated from the other primary toxin (amantin) generated by the toadstool in the 1940s, phalloidin is a bicyclic peptide composed of seven amino acids that specifically binds to and stabilizes junctions between subunits of filamentous actin, essentially interfering with the normal actin activity within cells. However, despite the change in F-actin's dynamic properties caused by bonding with phalloidin, the structure of the actin itself is not altered. Thus, fluorescent phalloidin conjugates are well suited for the needs of cell biologists who want to visualize actin filaments for fluorescence microscopy. The baby hamster kidney (BHK-21) fibroblasts featured in the digital image above were resident in a culture immunofluorescently labeled for microtubules with primary anti-tubulin mouse monoclonal antibodies followed by goat anti-mouse Fab fragments conjugated to the cyanine dye, Cy3. In addition, the cells were labeled with Alexa Fluor 488 conjugated to phalloidin and DAPI, targeting the filamentous actin network and nuclear DNA, respectively. Images were recorded in grayscale with a QImaging Retiga Fast-EXi camera system coupled to an Olympus BX-51 microscope equipped with bandpass emission fluorescence filter optical blocks provided by Omega Optical. During the processing stage, individual image channels were pseudocolored with RGB values corresponding to each of the fluorophore emission spectral profiles. |
© 1995-2022 by Michael W. Davidson and The Florida State University. All Rights Reserved. No images, graphics, software, scripts, or applets may be reproduced or used in any manner without permission from the copyright holders. Use of this website means you agree to all of the Legal Terms and Conditions set forth by the owners.
This website is maintained by our
|