Interactive Java Tutorials
Absorption Filters
Absorption filters, commonly manufactured from dyed glass or pigmented gelatin resins, are one of the most widely used types of filter for brightfield and fluorescence microscopy. These filters operate by attenuation of light through absorption of specific wavelengths, so that spectral performance is a function of the physical thickness of the filter and the amount of dye present in the glass or gelatin matrix. This interactive tutorial explores how absorption filters pass certain wavelengths of light while blocking others.
The tutorial initializes with a virtual absorption filter set to a wavelength passband of 130 nanometers, which is controlled using the Bandwidth slider. The filter bandwidth size can be varied between 1 and 200 nanometers to demonstrate the wide spectrum of absorption filters commercially available for optical microscopy. Above the sliders is a visible light spectral graph of the filter characteristics, which displays filter transmission percentage as a function of wavelength and automatically calculates the center wavelength (CWL) and full width at half maximum (FWHM) for all of the bandpass filters in this tutorial. Filter neutral density can be adjusted between zero and 100 percent with the Transmission slider. As this slider is translated to higher densities (lower transmission values), the intensity of light passing through the filter diminishes and the bandpass peak on the graph is reduced in amplitude. The Filter Wavelength Maximum slider is utilized to adjust the bandpass center (center wavelength; CWL) of the virtual filter. As this slider is translated, the center wavelength can be varied between 350 and 750 nanometers. The filter drawing in the upper portion of the tutorial simulates the spectrum of visible of light passing through the virtual absorption filter.
Until the early twentieth century, liquid filters and large blocks of dyed glass were the primary means of filtering light. Many aromatic organic chemicals produce brilliantly colored solutions when dissolved in alcohol or water, and these provided a wide range of absorption filters for early photographers and scientists. In 1856, English chemist William Perkin accidentally discovered a natural substance, termed aniline purple or mauveine, while attempting to synthesize the drug quinine from coal tar. He found that the chemical produced beautiful deep purple-colored solutions when dissolved in alcohol and realized its tremendous potential for making dye products. Perkin's efforts led to a host of synthetic dyes, which gave birth to an industry that is responsible for producing practically all of the dyes currently in use.
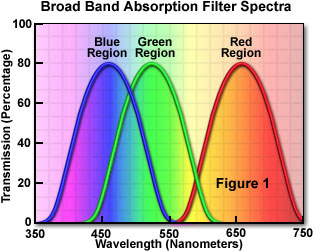
Today, absorption filters are made primarily from colored filter glass or synthetic gels, and represent the largest class and most widely used type of filters for applications that do not require a precise definition of transmitted wavelengths. Commonly utilized to isolate a broad band of wavelengths (see Figure 1), absorption filters are also helpful to block short wavelengths while transmitting longer ones. These filters are commonly available in the form of glass, plastic-coated glass, acetate, or gelatin bases that have been coated, mixed, or impregnated with organic and inorganic dyes obtained from both natural and synthetic sources. Among the materials used in glass and polymer filters are the rare earth transition elements, colloidal dyes (such as selenide), and other molecules having high extinction coefficients that produce reasonably sharp absorption transitions.
The quality of glass or polymer used in the manufacture of filters is important, and should be of optical grade and provide uniformity of density and color over the entire surface of the filter. Filter glass or plastic attenuates light only through absorption, so the spectral performance is dependent upon the thickness and optical density of the filter material. Increasing the thickness will produce a corresponding increase in the blocking level of unwanted wavelengths, but also reduces the peak in-band transmission, causing falloff at the ends of absorption bands.
Gelatin filters are the most cost effective and optically satisfactory filters available commercially, making this the filter material of choice for a wide variety of applications (including optical microscopy), despite the gentle handling required. Optical glass filters are also excellent, but these are generally not available to meet all of the consumer, industrial, or scientific applications. Acetate filters are generally useful for non-image forming applications where the need for quality and precision is unimportant. Typically, acetate filters are used in stage lighting, photographic enlargers, projection devices, and similar purposes. Plastic-coated filters are also limited in use to those applications suitable for acetate filters.
There are several advantages to glass and polymer absorption filters, including their relatively low cost and stability under a wide variety of climates and operating conditions. In addition, the filters are constructed with light-absorbing chemical species mixed throughout the filter material, rather than being deposited on the surface, so they are not prone to destruction by minor scratches or abrasions. Glass absorption filters are also resistant to chemical attack from corrosive oils in fingerprints and other sources of dangerous fumes and contamination, while polymer-based filters generally do not enjoy this immunity. Finally, glass and polymer filters are insensitive to the angle of incident illumination and provide uniform spectral characteristics, except for minor changes in absorption due to increased effective thickness when the filters are positioned away from the perpendicular.
The primary disadvantages of glass and polymer filters are their sensitivity to heat and susceptibility to altered light transmission properties upon prolonged use. There is also a limited selection of glasses available for those applications requiring specific optical-grade glass rather than polymer-based materials. Bandpass absorption filters generally possess poor slope characteristics when compared to interference filters, and often display low peak transmittance values. Also, because they depend upon thickness to dictate spectral performance, glass and polymer filters are less useful than other types of filters designed for specialized applications. In addition, most longpass filter glasses are plagued by high autofluorescence, which can sometimes be avoided by substituting polymer-based filters with lower levels of autofluorescence than their glass counterparts.
Contributing Authors
Mortimer Abramowitz - Olympus America, Inc., Two Corporate Center Drive., Melville, New York, 11747.
Matthew J. Parry-Hill, Ian D. Johnson, and Michael W. Davidson - National High Magnetic Field Laboratory, 1800 East Paul Dirac Dr., The Florida State University, Tallahassee, Florida, 32310.
