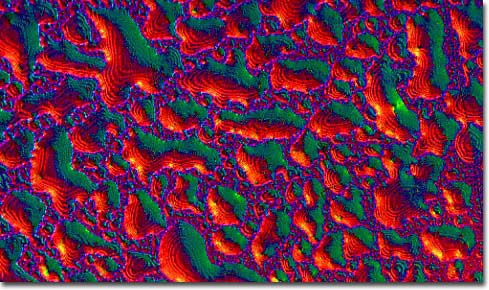
|
Fullerenes are a class of closed, hollow carbon compounds that comprise only the third form of pure carbon ever discovered. The most remarkable of the fullerenes is the 60-carbon alkene buckminsterfullerene, also known as a Buckyball. This highly unusual molecule was named after the geodesic dome, a structure that exhibits a geometry that approximates a truncated icosohedral soccerball-shape, invented by visionary engineer, author, and architect R. Buckminster Fuller. The roundest known molecule in the world, the Buckyball has carbon atoms at 60 chemically equivalent vertices that are connected by 32 faces, 12 of which are pentagonal and 20 hexagonal. Due to their unique structure, Buckyballs are remarkably rugged, being capable of surviving collisions with metals and other materials at speeds in excess of 20,000 miles per hour, a pace that would tear most organic molecules apart. Higher and lower order Buckyballs containing different numbers of carbon atoms deviate from the strict geodesic dome structure and are consequently not as stable, but are still composed primarily of pentagons and hexagons. Smaller fullerenes, often termed Buckybabies, are said to have a shape akin to that of asteroids, while larger fullerenes may appear similar in shape to a pentagon.
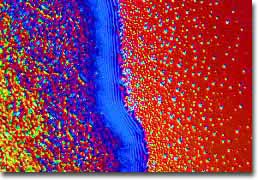
Edge of a C-60 Thin Film
The discovery of fullerenes, which exhibit a number of notable characteristics that make the possibilities of their use seem almost limitless, was an important moment in the history of science. This achievement is generally attributed to Richard Smalley and Robert Curl of Rice University as well as Harold Kroto of the University of Sussex, whose experiments aimed at understanding the mechanisms by which long-chain carbon molecules are formed in interstellar space serendipitously produced Buckyballs in the mid-1980s. In 1996, Smalley, Curl, and Kroto were awarded the Nobel Prize in Chemistry for their discovery. Yet, the discovery of fullerenes did not necessarily mean that there was a readily available way to study them. For a time, much of the work regarding fullerenes was chiefly theoretical since a practical means of producing significant amounts of the molecules was unknown until 1988, when astrophysicists Donald Huffman and Wolfgang Kratschmer realized that they had already discovered a way to produce them years before, though at that time they did not know the import of their actions. The Huffman/Kratschmer process soon made it possible to generate isolable quantities of fullerenes by causing an arc between two graphite rods to burn in a helium atmosphere and extracting the carbon condensate that resulted utilizing an organic solvent.
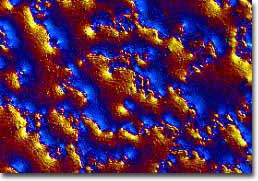
C-60 Thin Film
In 1991, Science magazine dubbed the Buckyball "molecule of the year," professing it "the discovery most likely to shape the course of scientific research in the years ahead," a statement that, years later, does not appear unsubstantiated. Studies exploring the extraordinary characteristics of Buckyballs and potential uses for them are ongoing and the molecules may eventually wind their way into daily life as practical applications are developed. One of the most promising areas of Buckyball research is in the realm of materials science, many scientists believing that the extremely stable molecules could yield new and improved lubricants, protective coatings, and other materials. But, even more exciting to some are the possible materials that may be produced by combining the carbon framework of the Buckyball with different atoms. The process of knocking one or more carbon atoms out of the Buckyball structure and replacing it with metal atoms is known as doping, and the molecule in its altered form is often referred to as a dopeyball. The electrical and magnetic properties of dopeyballs have been the subject of intense study, which has already resulted in the discovery that potassium-doped Buckyballs are capable of superconducting at 18 K and those doped with rubidium superconduct at 30 K.
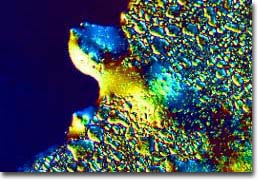
Edge of a C-60 Thin Film
In addition to doping Buckyballs with other atoms, the hollow structure of the geodesic molecules makes it possible to trap atoms inside them like a molecular cage. This strange capability of Buckyballs has caught the attention of the medical community. Indeed, many researchers believe that eventually Buckyballs may be used to deliver medicines to specific tissues and cells, such as those that have been attacked by bacteria, viruses, or cancer, protecting the rest of the body from the toxic effects of potent pharmaceuticals. This same concept is currently being used to develop improved Medical Resonance Imaging (MRI) contrast agents and image enhancers that exploit the carbon cage of a Buckyball to shield patients from the radioactive materials inside. There are also many non-medical possibilities for atom-filled Buckyballs, which are termed endohedral metallofullerenes (EMFs) when the atoms trapped inside are metallic. For instance, EMFs are well on their way to being utilized in organic solar cells and may one day be crucial components of nanoelectronic devices, which many predict will eventually revolutionize the modern communications industry. Some EMFS have also shown potential for use as chemical catalysts that could be delivered to support surfaces in novel ways.
C-60 Thin Film
What is perhaps most amazing about Buckyballs is that despite their circuitous discovery in the laboratory, they have been naturally present on Earth all along. The earliest evidence that Buckyballs occur in nature was discovered by Arizona State University researchers Semeon Tsipursky and Peter Buseck, who found that a sample of rare, carbon-rich rock called shungit, estimated to have been formed between 600 million and 4 billion years ago, contained both C60 and C70 fullerenes. Since then, the fascinating molecules have also been identified in meteorites, impact craters, and materials struck by lightning. This new information has led some scientists to speculate about the role that fullerenes may have played in the development of life on Earth. Indeed, gases are known to become readily trapped inside the hollow molecules, and one research group has already found evidence of a form of helium in Buckyballs taken from the Sudbury crater (which contains the greatest known concentration of fullerenes in the world) that likely originated not inside of our own solar system, but within a red giant star. Thus, many consider it to be theoretically possible that Buckyballs carried from their stellar origin both the carbon essential to life and the volatiles that helped produce the planetary conditions necessary for life to begin.
|