
Spin-Dependent Reserve Batteries
Spin-Dependent reserve batteries are used for many military and other applications, and are characterized by their long shelf life.
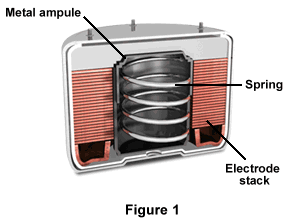
As illustrated in Figure 1, each battery has a metal ampoule, a spring, and an electrode stack for both the anode and cathode. The electrolyte is in the stack's center, and resides inside a metal ampoule. The battery is packaged dry, which results in long-term storage capability. Electrode stacks for spin-dependent batteries consist of electrodes and cell spacers that have annular configurations.
Certain battery-requiring systems, especially military systems, require that batteries be stored and packaged with the electronics. These applications, such as spin-stabilized projectiles and fusing, fare badly with batteries that need to be replaced during their own storage life. Often, it is difficult to create a battery for an environment of high spin forces, including one for artillery projectiles. Recently, however, unique designs of batteries have actually used spin to their advantage, and utilize it for their activation.
ChemistryThe most common spin-dependent reserve batteries use a lead/fluoboric acid/lead dioxide cell. Fluoboric acid is used instead of sulfuric acid as the electrolyte because it performs very well at low temperatures, which is often required in military applications. In the last decade, spin-dependent reserve batteries have employed lithium anodes and have used thionyl chloride for both electrolyte carrier and cathodic depolarizer. Before the creation of the lithium anode, and still in some cases where there is potential danger, a zinc/potassium hydroxide/silver oxide system is utilized. A current example of this version of a spin-reserve battery is used for war missiles, where there is no spin application involved, and the electrolyte is shot into its place by a gas generator.
There are two types of electrode stacks, one for high voltage, which uses bipolar electrodes and positions the anode and cathode materials on opposite sides of a metal substrate, and the other for high current.
The spin-dependent reserve battery's periphery must be sealed because of its annular shape; otherwise the electrolyte has the potential to leak. A plastic block usually forms around the outside of the electrode-spacer stack. Fish paper replaces the plastic block for lead/fluoboric acid/ lead dioxide batteries.
Early liquid-electrolyte reserve batteries used glass ampoules to hold the electrolyte. In certain applications, especially those where a gun is fired, a glass ampoule has the potential to break. When this happens, the battery no longer works as a result of leakage of electrolyte. Spin-dependent reserve batteries utilize a metal ampoule complete with internal cutting mechanisms, some which are activated by spin.
Additional InformationTemperature affects liquid-electrolyte reserve batteries. Lead/lead dioxide/fluoboric acid systems and lithium/thionyl chloride and zinc/potassium hydroxide/silver oxide systems are able to operate between -40 to 60 degrees Celsius. In addition, voltage is a lot lower at low temperatures than at high temperatures, a problem that thermal batteries can fix. The storage life of a spin dependent reserve battery depends on the temperature at which it is stored, with high temperatures resulting in shorter life. Zinc/silver oxide cells, the most vulnerable, usually have a ten-year life span. Lithium/thionyl batteries were developed too recently to know how long their shelf life is, but scientists approximate a long potential.